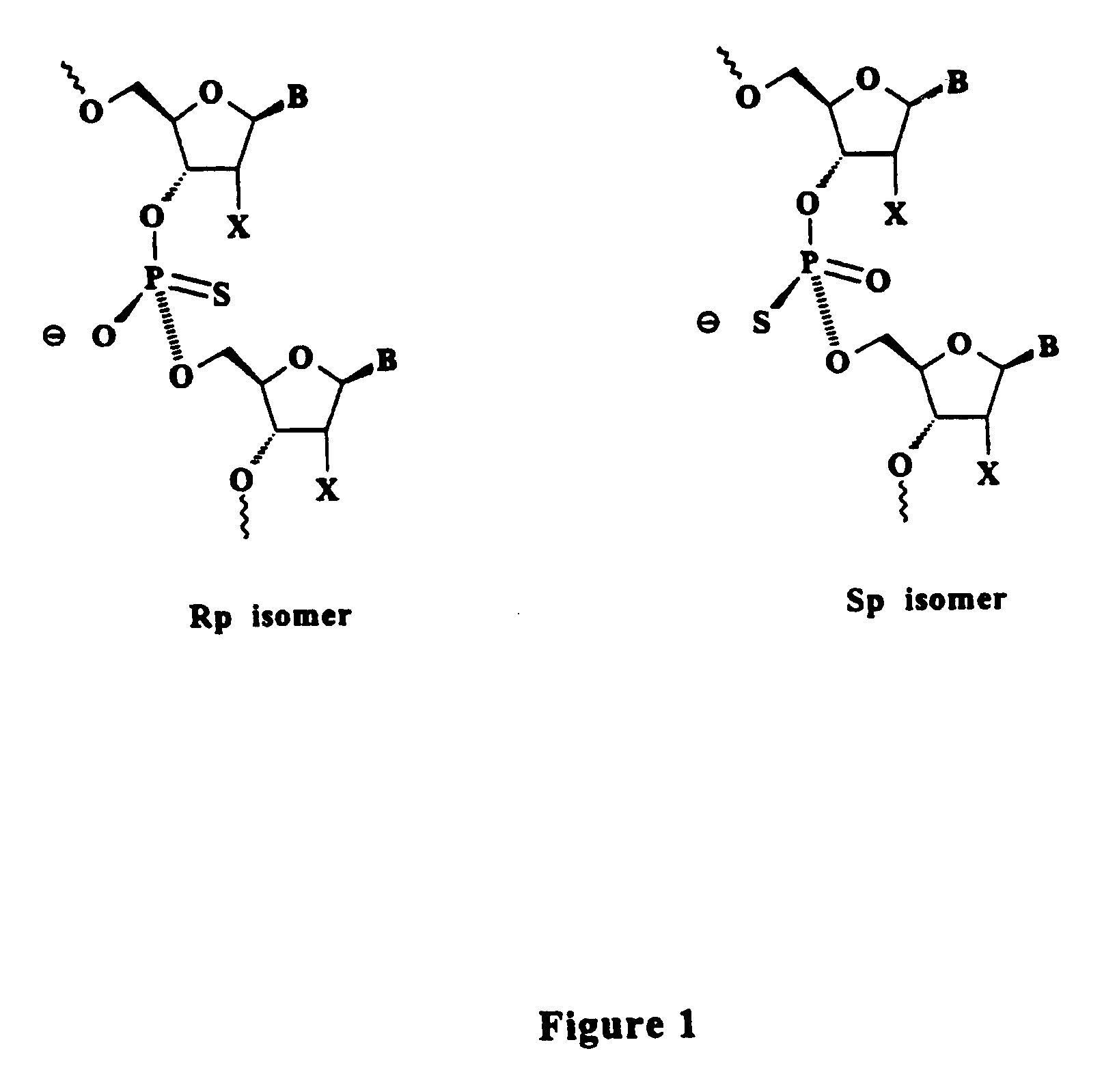
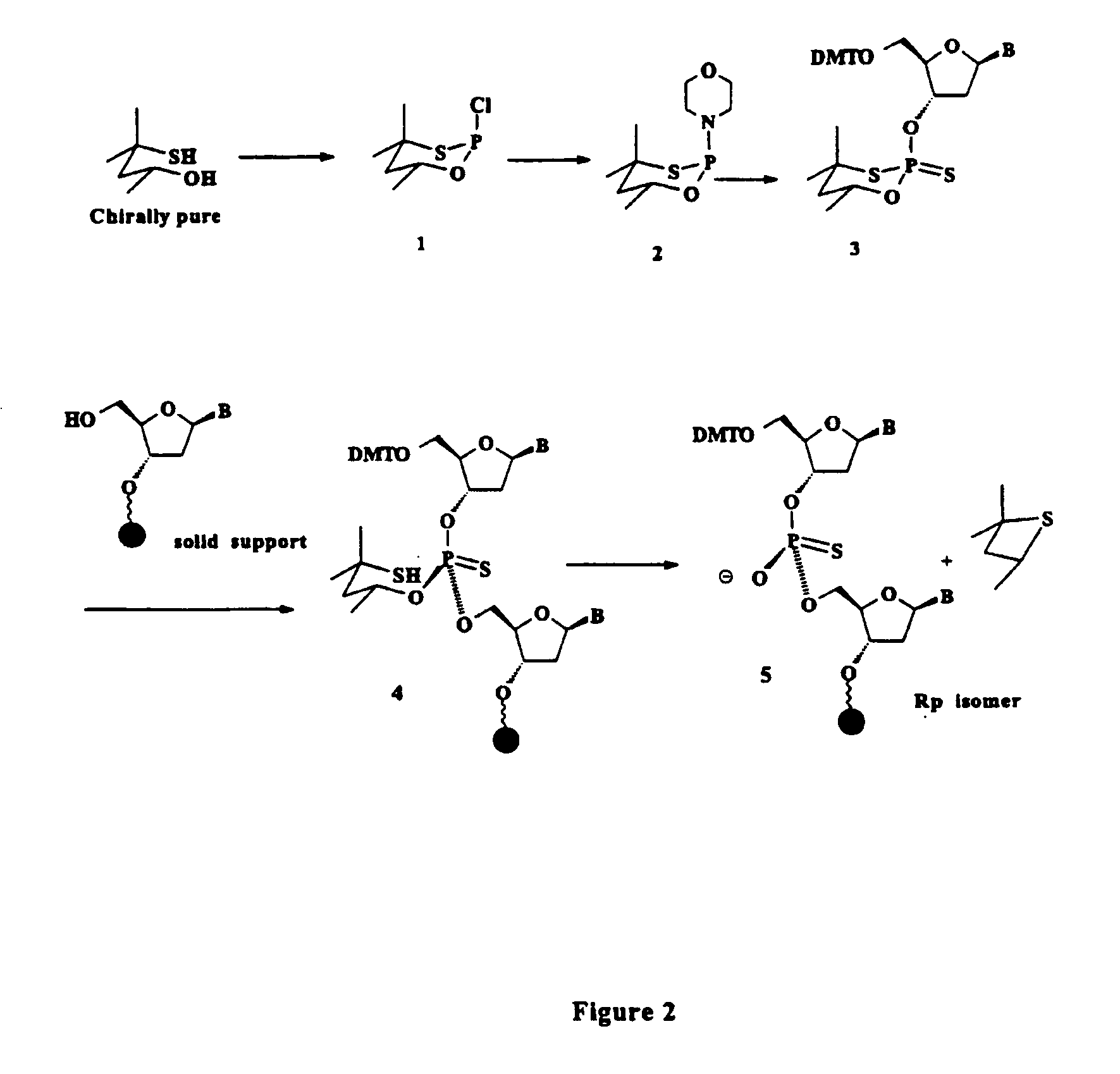
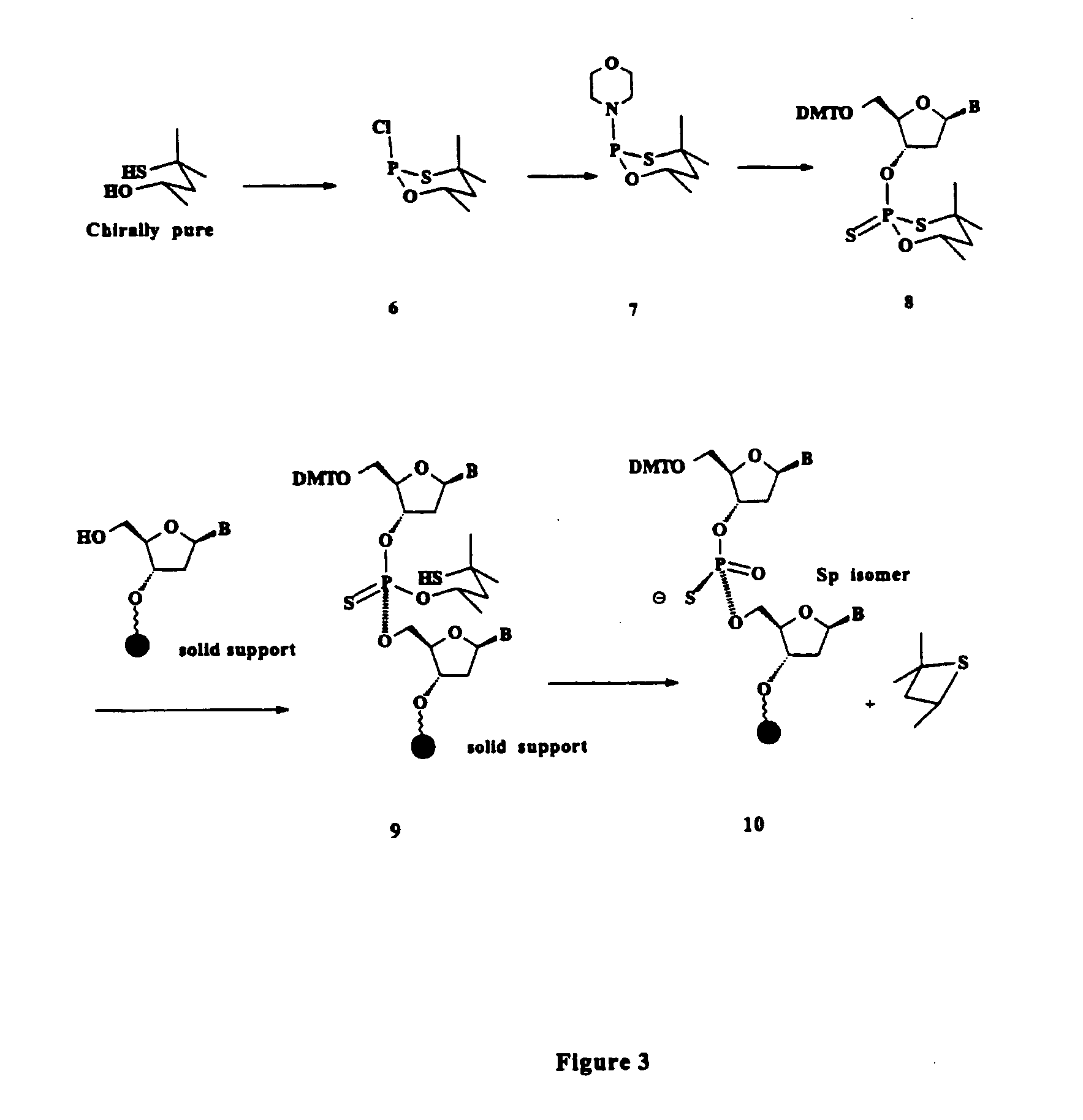
Oligonucleolotides having site specific chiral phosphorothioate internucleoside linkages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isomerically Pure R and S Isomers of 4-mercapto-4methyl-2-pentanol
[0261]R4-mercapto-4-methyl-2-pentanol and S-4-mercapto-4-methyl-2-pentanol are synthesized according to the procedure of Eliel and Morris-Natschke (Eliel, E. L., Morris-Natschke, S., J.Am.Chem.Soc, 1984, 106, 2937-2942).
example 2
Rp Precursor, Compound 1
[0262]PCl3 (1.3 mL, 15 mmol) is introduced via a syringe into a dry 100-mL round-bottomed flask containing 20 mL of dry THF that has been flushed with argon and sealed with a septum. The flask is cooled to −78° C. in a dry ice / acetone bath, and a solution of (R)-4-mercapto-4-methyl-2-pentanol (15 mmol) in THF (15 mL) containing triethylamine (6.9 mL, 50 mmol) is added via a syringe. The reaction mixture is stirred for 30 min at −78° C. and then warmed to 0° C. for 1 hour. The reaction mixture is partitioned between CH2Cl2 and saturated NaHCO3 and washed with saturated NaCl and dried over anhydrous Na2SO4 to give the title compound.
example 3
Compound 2
[0263]Compound 1 in hexane is treated with morpholine by careful dropwise addition at 0° C. The cold bath is removed, and the mixture is stirred at room temperature for an additional 1 hour. Morpholine hydrochloride is removed by filtration, and Compound 2 is purified by silica gel column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com