Pro-angiogenic peptides and uses thereof
a technology of peptides and peptides, applied in the field of therapeutic peptides and endogenous cytokine modulation, can solve the problems of affecting the healing process, affecting the quality of life, and causing deleterious inflammation, so as to reduce the expression of at least one harmful cytokine, and modulate the expression of cytokin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Peptide Design and Synthesis
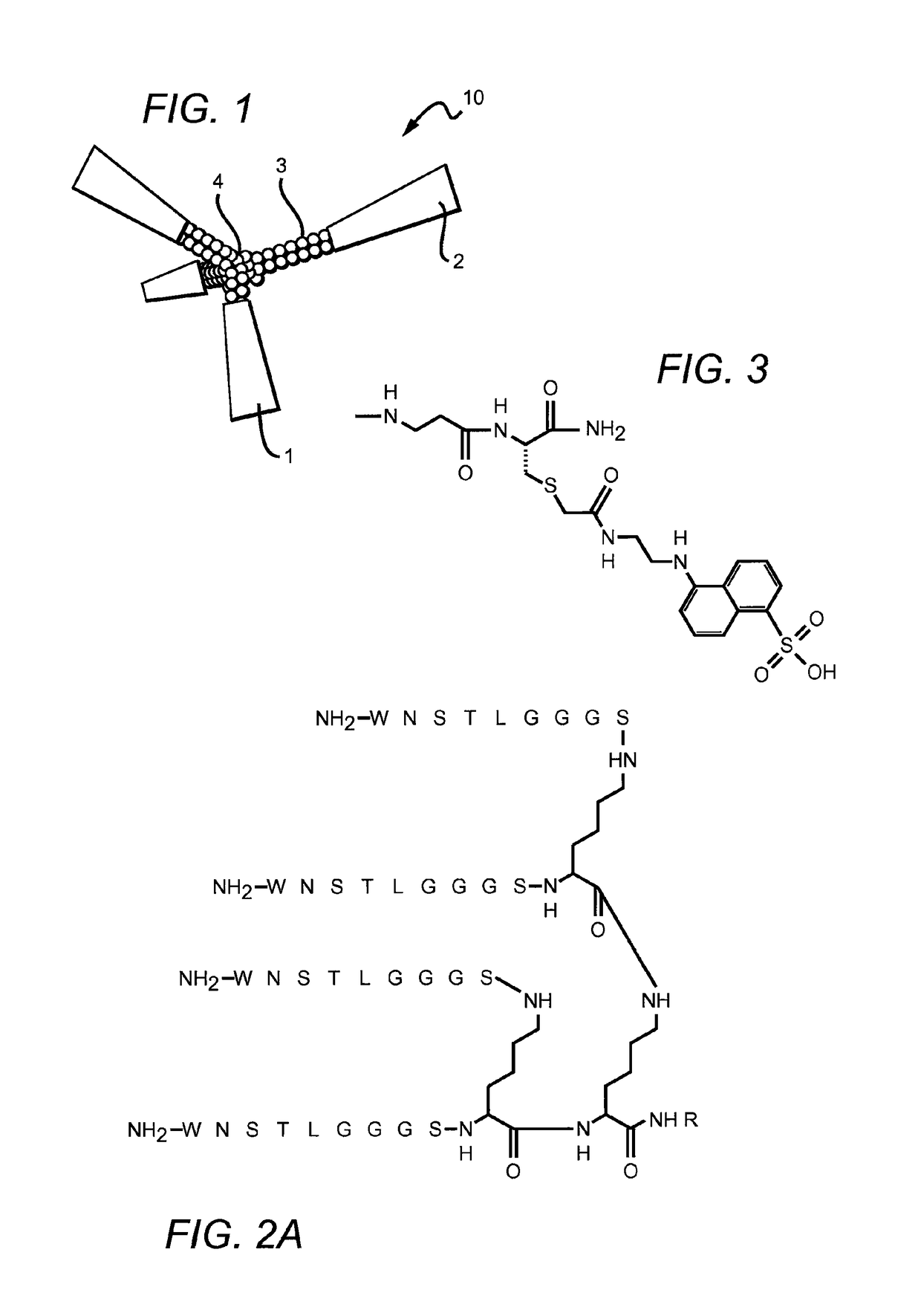
[0103]A screen of peptide sequences identified one set of sequences of interest. The corresponding peptides were synthesized by solid-phase methods using standard Fmoc side chain protection. Branched peptides were constructed on a central tri-lysine framework, which allows four identical sequences within the same structure. A (Gly)3-Ser (GGGS, SEQ ID NO:3) linker sequence was included to distance the active sequence from the central framework. Distances between the active sequences can be adjusted by decreasing or increasing the length of the linker, including without limitation the use of two linkers in tandem (GGGSGGGS, SEQ ID NO:4) or by inserting any inert linker such as polyethylene glycol (PEG) of a variable length. The branched structure was designed to have enhanced activity by causing receptor clustering (cross-linking) on the surface of responsive cells.
[0104]The peptides were synthesized on PAL-PEG-polystyrene resin (Applied Biosystems, Foster ...
example 2
Core Peptide Sequences: WNSTL (SEQ ID NO:1) and NQHTPR (SEQ ID NO:2)
[0110]The peptides (WNSTL, SEQ ID NO:1) and (NQHTPR, SEQ ID NO:2) were identified through a screen of peptide sequences as potentially of interest. The sequences were synthesized on a tri-lysine core according to Posnett et al., J. Biol. Chem., 263: 1719-25, 1988, with a linker (GGGS, SEQ ID NO:3) included with the sequence to extend the active peptide away from the core.
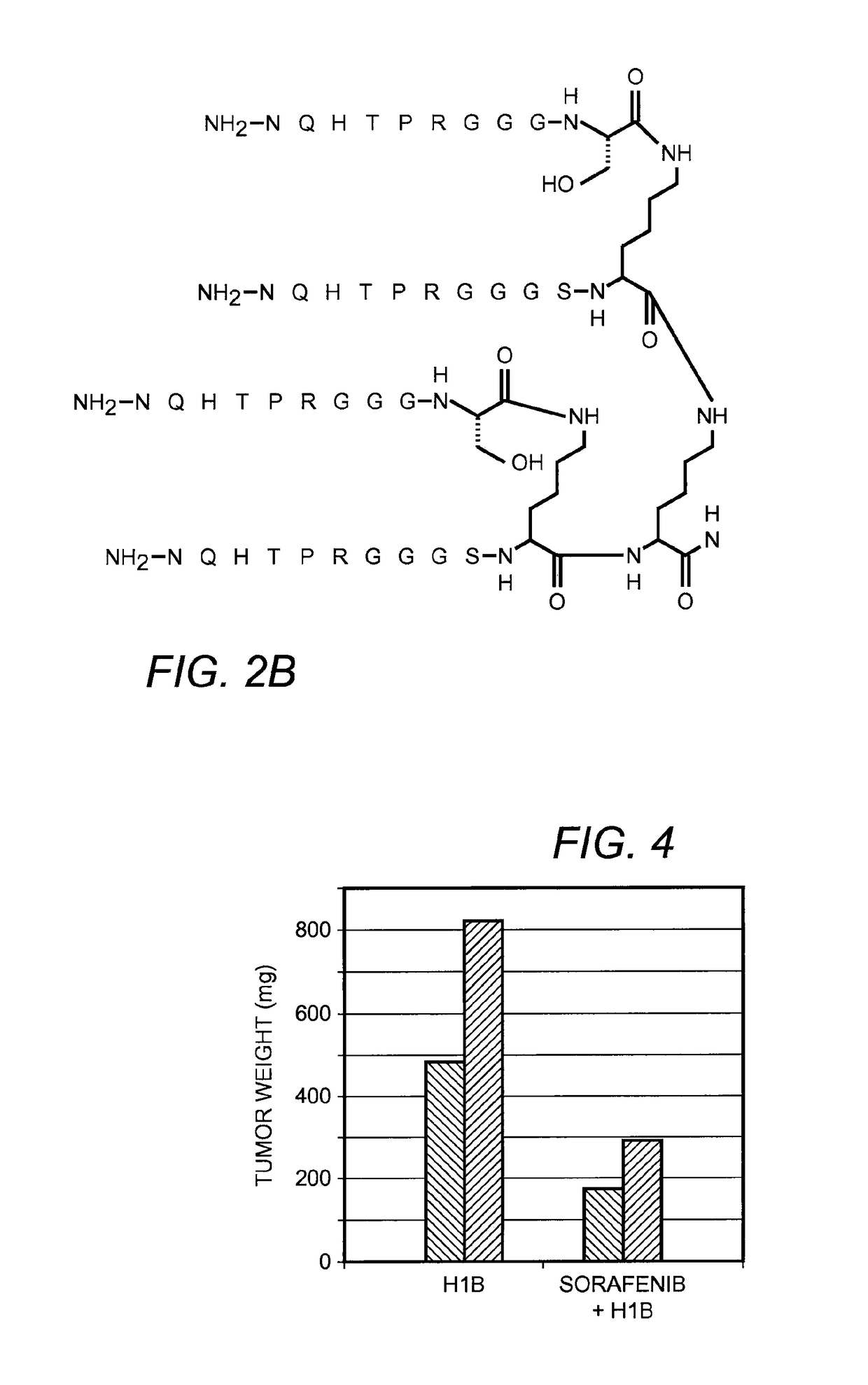
[0111]FIGS. 2A-B illustrate the chemical structure of these two embodiments of the invention. In these embodiments, R═H or can be an adduct containing a fluorescent tag such as the dansyl group shown in FIG. 3. The peptide constructs illustrated contain four identical sequences, each of which is connected to a branched central tri-lysine framework via a (Gly)3Ser (GGGS, SEQ ID NO:3) linker. FIG. 2A is a branched peptide construct according to one embodiment of the invention, a peptide construct which contains four copies of the core sequence WNSTL (...
example 4
Pro-Angiogenic Activity of Peptides
[0147]Tumors require vascularization to obtain nutrients to support growth. Therefore, stimulation of growth of a tumor in response to administration of a construct of this invention is an indication of angiogenesis. For this example, a xenograph model system with the nude mouse (nu / nu) was used to determine the effect of peptide on growth of 786-0 human renal cell adenocarcinoma cell line injected into the flank of a mouse so as to induce a tumor. After the tumor was established, peptide was injected subcutaneously on alternate days. The weight of the tumor was estimated by calculation of the volume.
[0148]FIG. 4 illustrates data resulting from assays for pro-angiogenic activity for one embodiment of the invention. The bar graph in FIG. 4 shows the average weight of the tumor in mice treated with the peptide construct containing four copies of the core sequence WNSTL (SEQ ID NO:1) at a dose of 0.05 nmole / gm as compared with a control group. The pep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com