Ophthalmic drug delivery device
A technology for releasing devices and drugs, applied in the field of implants, can solve problems such as increased bleeding and accumulation of subretinal fluid, increased blood flow of choroidal hyperemia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] The preferred embodiment of the present invention and its advantages are best understood by referring to Figures 1 to 21 of the accompanying drawings, wherein like reference numerals will be used for like or corresponding parts in the various figures.
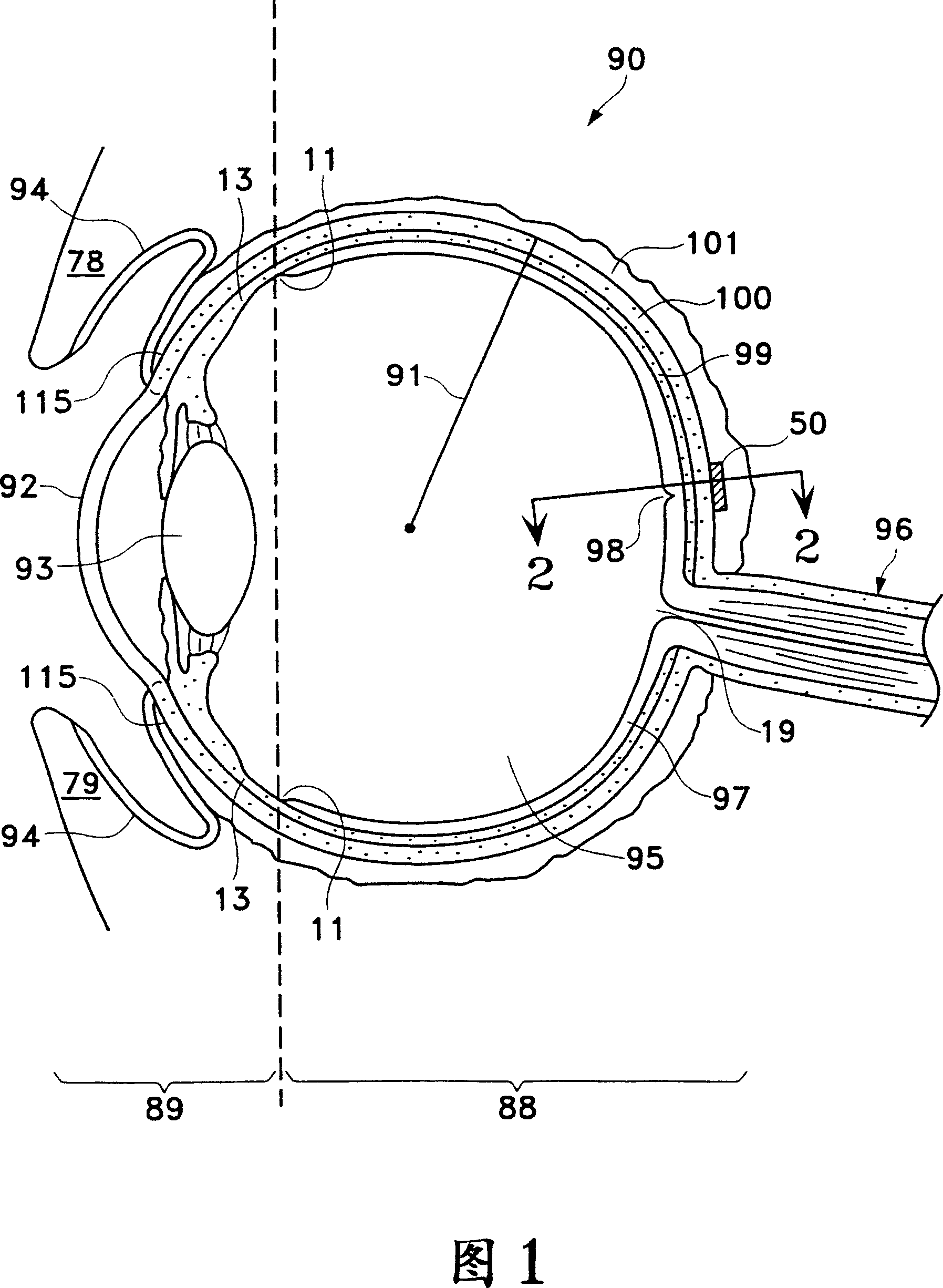
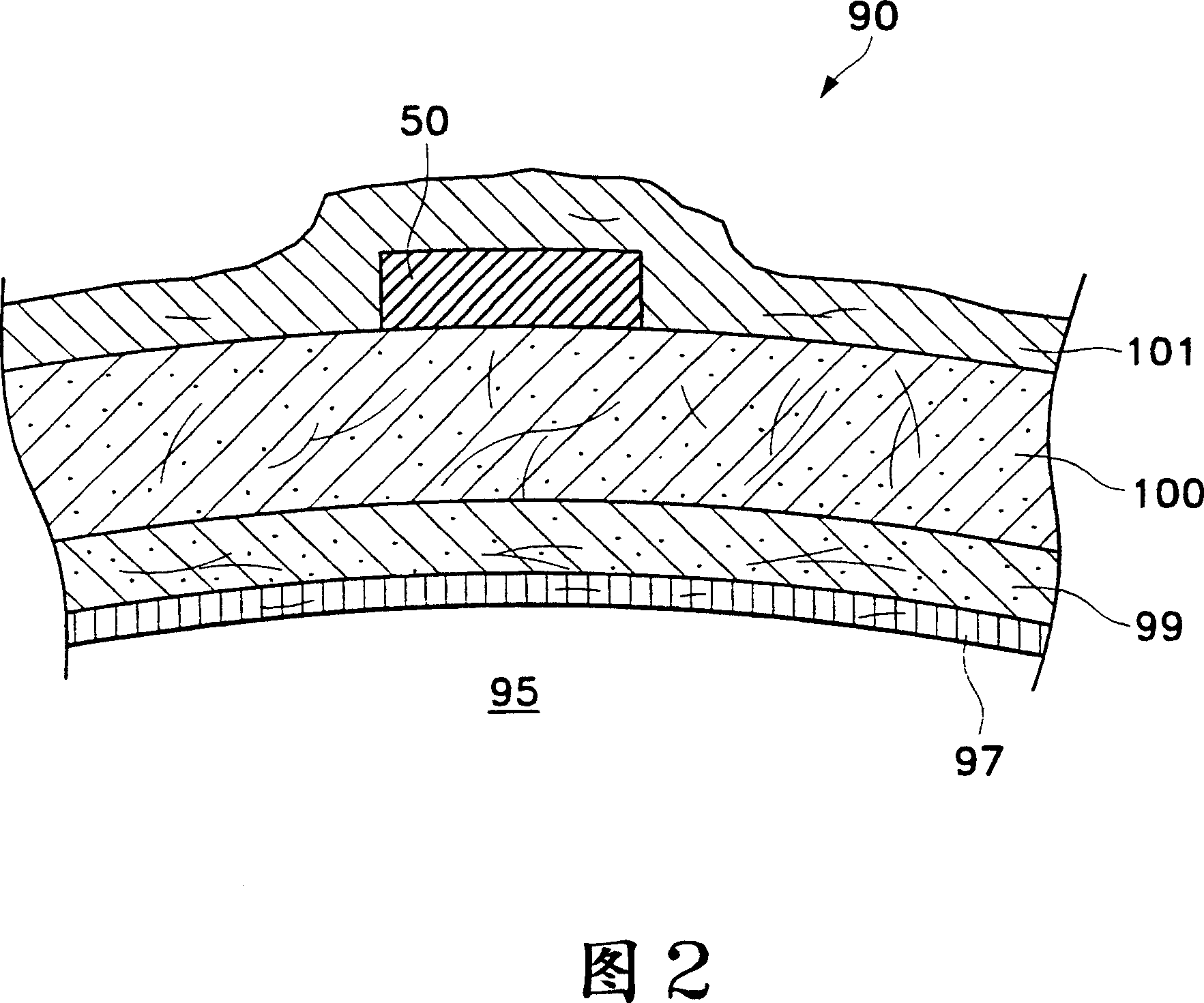
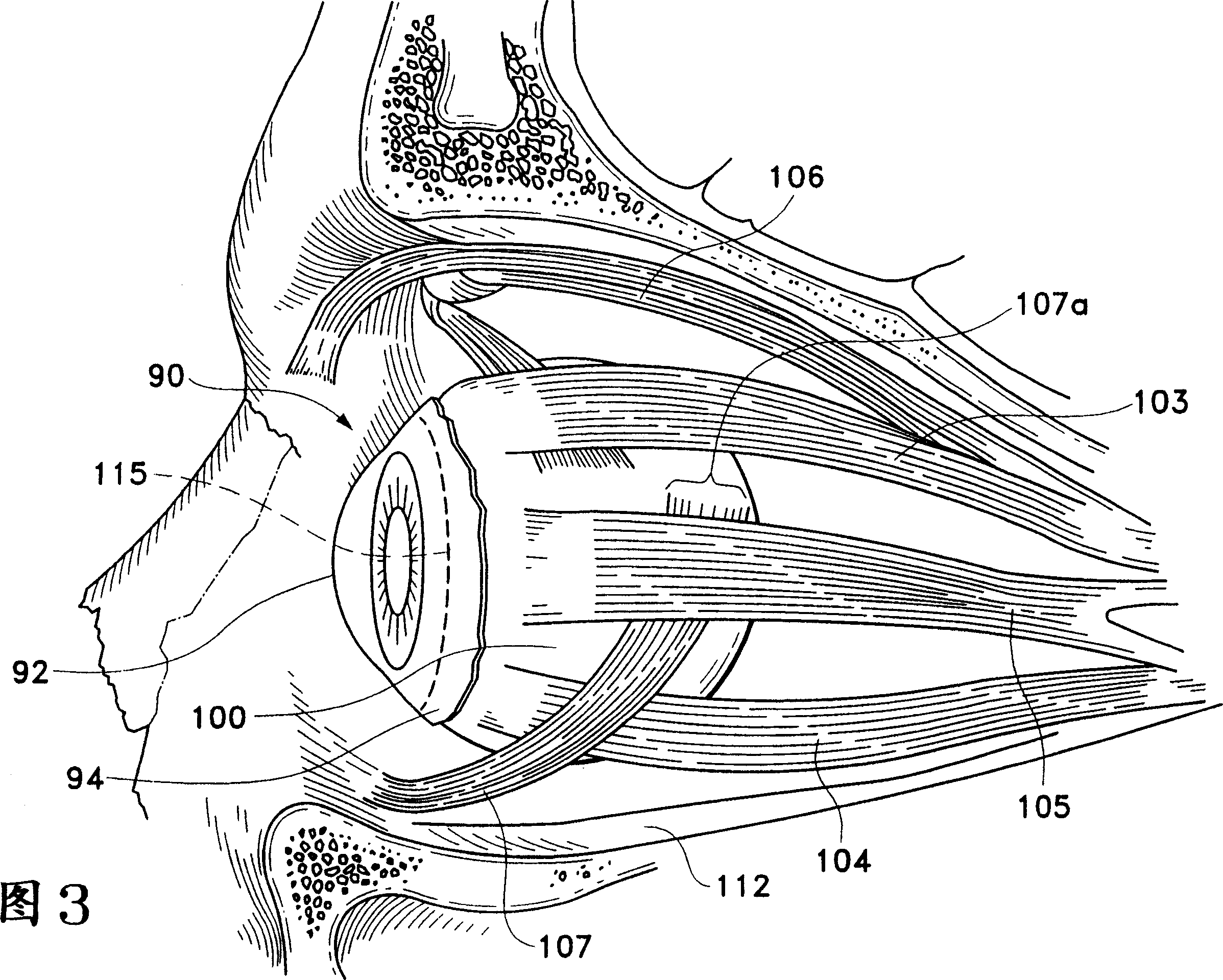
[0037]Figures 1 to 6 illustrate parts of the human eye that are important to understanding the present invention. Referring to Figure 1, a human eye 90 is schematically shown. Eye 90 has a cornea 92 , a lens 93 , vitreous body 95 , a sclera 100 , a choroid 99 , a retina 97 and an optic nerve 96 . The eye 90 is generally divided into an anterior portion 89 and a posterior portion 88 . Front portion 89 of eye 90 includes the portion of eye 90 that is forward of ora serrata 11 . The rear portion 88 of the eye 90 generally includes the portion of the eye 90 behind the ora serrata 11 . The retina 97 is physically connected to the choroid 99 in a circumferential manner, proximal to the ciliary annulus 13 behind the optic di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com