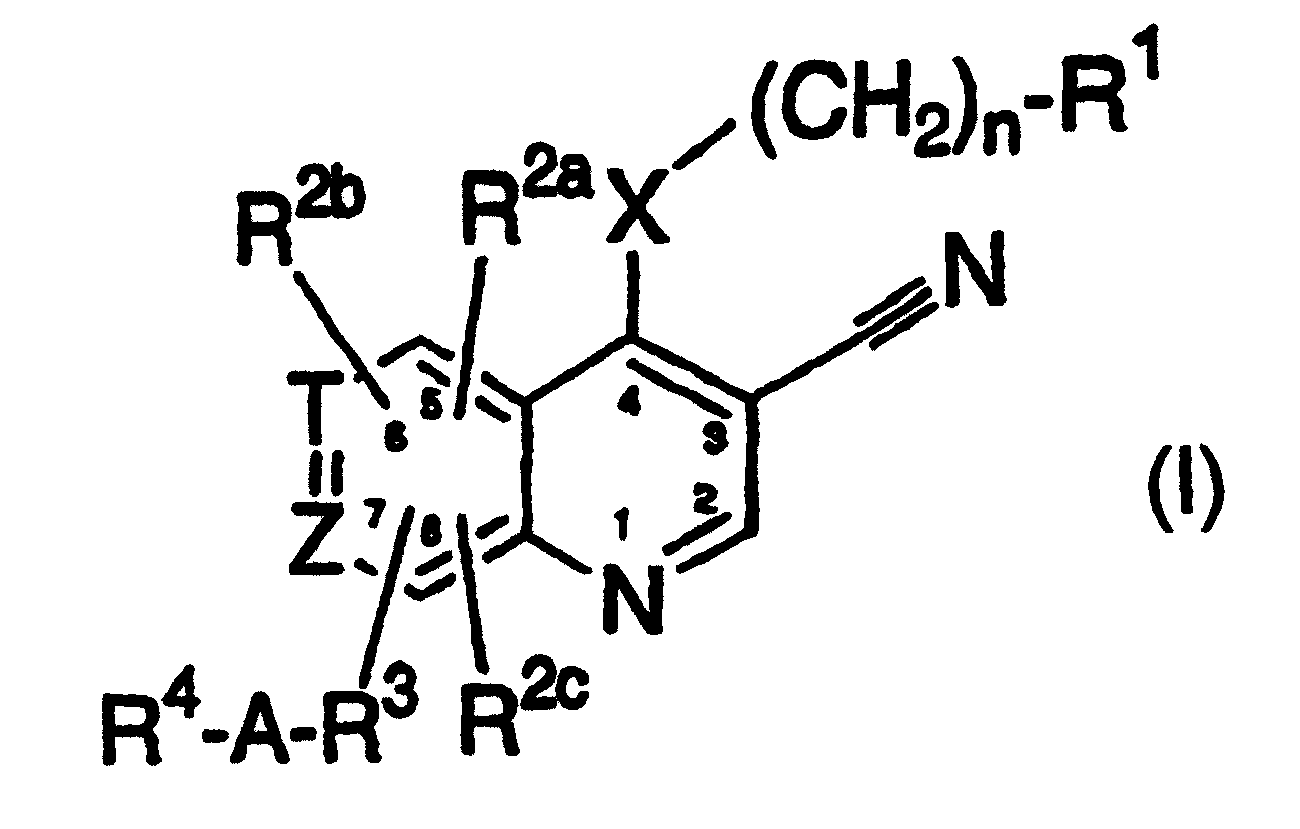
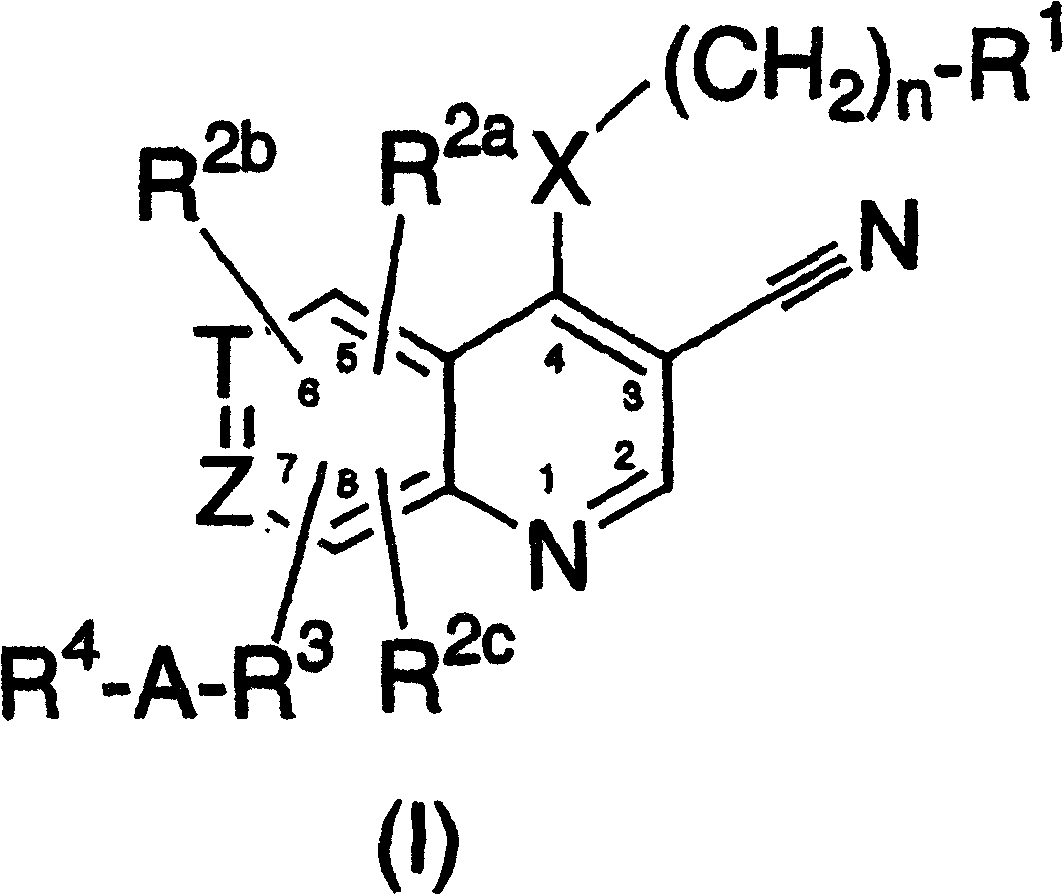
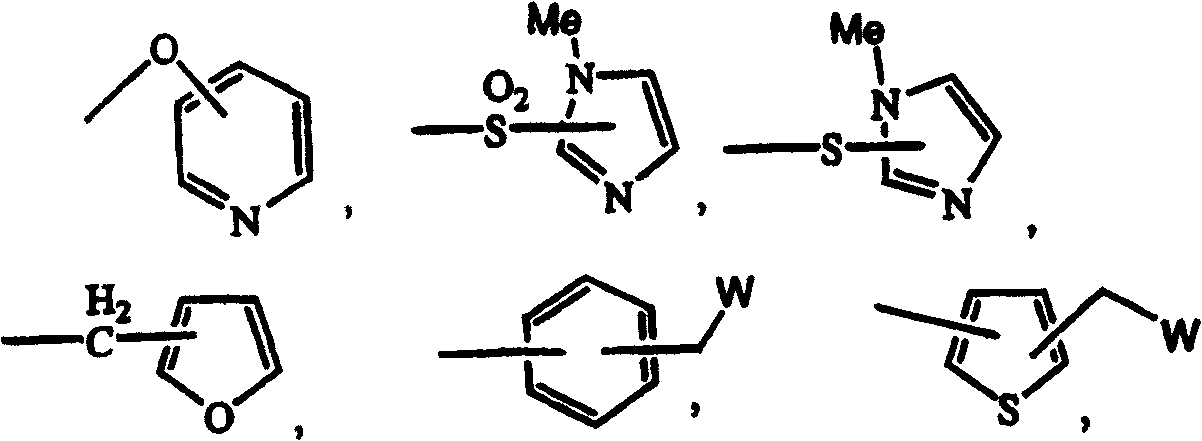
3-cyanoquinolines, 3-cyano-1,6-naphthyridines, and 3-cyano-1,7-naphthyridines as protein kinase inhibitors
A group, cycloalkyl technology applied to 3-cyanoquinoline, 3-cyano-1,6-naphthalene and 3-cyano-1,7-diazine as protein kinase inhibitors In the field of azanaphthalenes, problems such as undisclosed 3-cyano groups can be solved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0976] 6-Bromo-4-oxo-1,4-dihydroquinoline-3-carbonitrile
[0977] A solution of 5-bromoanthranilic acid (21.6g, 100mmol) and dimethylformamide dimethyl acetal (50ml) in dimethylformamide (150ml) was heated at 155-160°C for 8 hours, then cooled to room temperature. Volatiles were removed under vacuum to yield 28.5 g of the intermediate amidine.
[0978] Lithium diisopropylamide (LDA) was produced from isopropylamine (9.84ml, 70.2mmol) and 2.5M n-butyllithium (29.5ml, 70.2mmol) in tetrahydrofuran (150ml) at -78°C. Acetonitrile (3.67ml, 70.2mmol) was added and the resulting white suspension was stirred at -78°C for 1 hour. A solution of 10 g of the above-mentioned amidine in 100 ml of tetrahydrofuran was added and stirring was continued for 1 hour at -78°C and then for 1 hour at room temperature. Acetic acid (15ml) was added to quench the reaction. Volatiles were removed under vacuum and water was added to the residue. Ammonium hydroxide was added to basify the aqueous sol...
reference example 2
[0984] 6-Bromo-4-chloro-3-quinolinecarbonitrile
[0985]A mixture of 6-bromo-4-oxo-1,4-dihydroquinoline-3-carbonitrile (1.3 g, 4.86 mmol) and 8 ml of phosphorus oxychloride was heated at reflux for 30 minutes. The resulting dark brown solution was cooled to room temperature and 10 ml of hexane was added. The resulting solid was collected by filtration, washed with hexane, water and hexane to give 1.05 g of 6-bromo-4-chloro-3-quinolinecarbonitrile as a tan solid.
[0986] 1 H NMR (DMSO-d 6 )δ8.12(d, J=9Hz, 1H), 8.19(dd, J=9, 2Hz, 1H), 8.45(d, J=2Hz, 1H), 9.23(s, 1H); MS(ES)m / z 267.1, 269.0(M+1).
[0987] Elemental Analysis: C 9 h 4 BrClN 2 :
[0988] Calculated: C, 44.90; H, 1.51; N, 10.47.
[0989] Found values: C, 44.53; H, 1.63; N, 10.27.
reference example 3
[0991] 6-bromo-4-(2,4-dichloro-5-methoxyanilino)-3-quinolinecarbonitrile
[0992] A mixture of 2,4-dichloro-5-methoxyaniline (prepared as described in WO 8501939-A1) (730 mg, 3.77 mmol) and sodium hydride (180 mg 60% oil dispersion, 4.5 mmol) in 30 ml THF Heat at reflux for 1 hour. The mixture was cooled, 6-bromo-4-chloro-3-quinolinecarbonitrile (600mg, 2.24mmol) was added, and the resulting mixture was heated under reflux for 50 minutes. After cooling to room temperature, the reaction mixture was partitioned between ethyl acetate and saturated sodium bicarbonate. The organic layer was washed with saturated sodium chloride, dried over magnesium sulfate, filtered and concentrated in vacuo. The obtained solid was purified by flash chromatography, and 3:1-1:1 hexane: ethyl acetate was used for gradient elution to obtain 530 mg (yield 53%) of 6-bromo-4-(2,4-di Chloro-5-methoxyanilino)-3-quinolinenitrile, melting point (mp) 232-234°C.
[0993] 1 H NMR (DMSO-d 6 / trifluoroac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com