Chewing tablet for discharging lead, and preparation method
A chewable tablet and lead mouth technology is applied in the field of health food and its preparation to achieve the effects of increasing lead discharge, preventing diseases, and having no toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
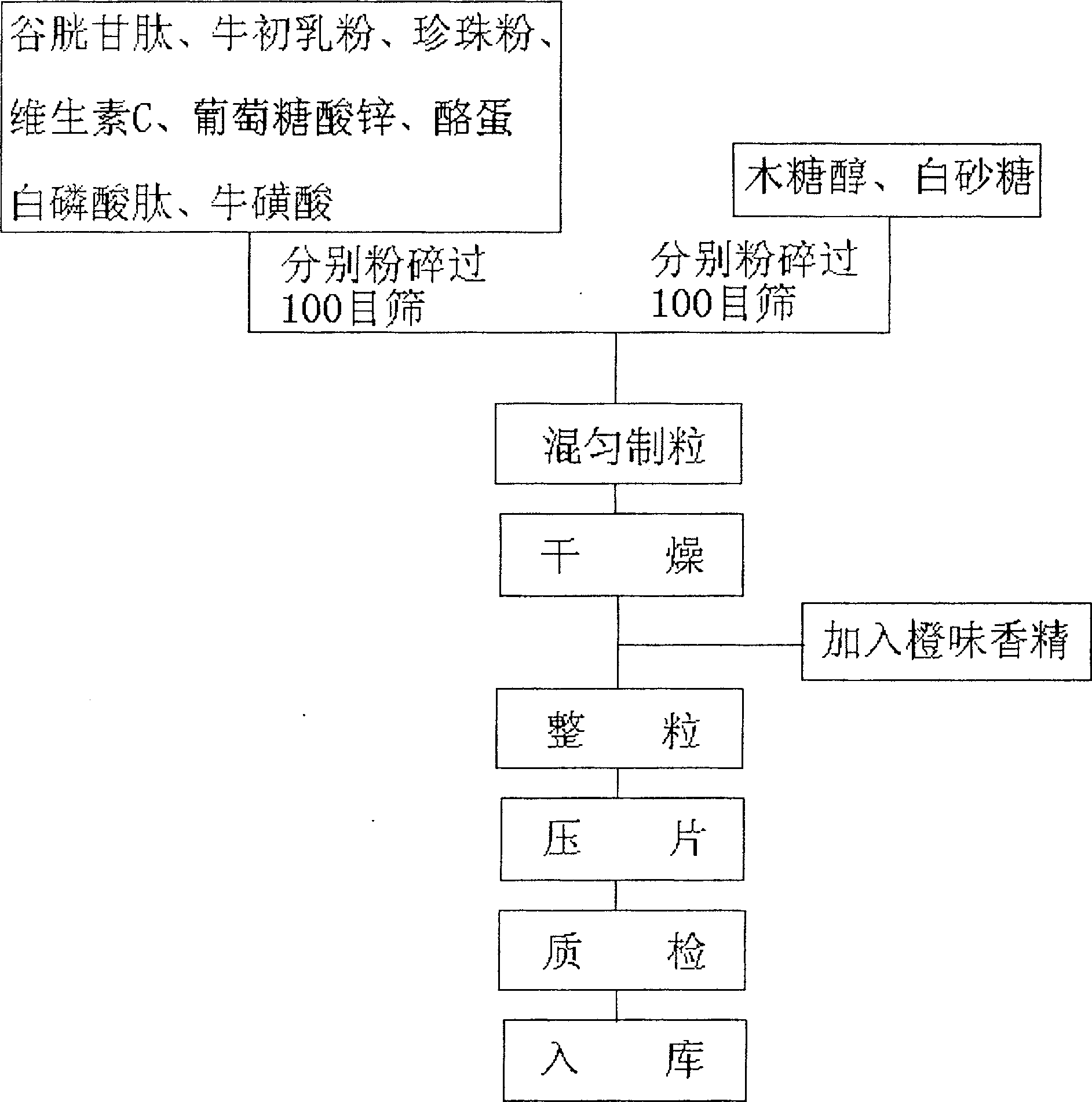
[0036] Embodiment 1: The chewable tablet for discharging lead of the present invention includes
[0037] Glutathione (purity not less than 98%) 248g
[0038] Bovine Colostrum Powder (Contains 608g Immunoglobulin) 1520g
[0039] Pearl powder 2002g
[0040] Vitamin C 503g
[0041] Zinc Gluconate (Zinc 26.24g) 202g
[0042] Casein phosphopeptide (purity not less than 85%) 127g
[0043] Taurine 53g
[0044]Xylitol 1500g
[0045] White sugar 1365g
[0046] Orange flavor 1.284g
[0047] A total of 7521.284g, each piece of 0.75g can be made into 10,000 pieces of qualified lead-discharging chewable tablets
[0048] [Eating method and dosage] Twice a day, two tablets each time, half for children.
[0049] [Storage method] Sealed in a cool dry place.
[0050] 1. Glutathione was purchased from Beijing Jingke Hongda Biotechnology Co., Ltd., which meets the requirements of Schedule 1;
[0051] 2. Pearl powder complies with the regulations of the 2000 edition of the Pharmacopoeia...
Embodiment approach 2
[0073] Embodiment 2: The chewable tablet for discharging lead of the present invention includes
[0074] Glutathione (purity not less than 98%) 102g
[0075] Bovine colostrum powder (contains 400 grams of immunoglobulin) 1000g
[0076] Pearl powder 2000g
[0077] Vitamin C 400g
[0078] Zinc Gluconate (Zinc 15g) 115.5g
[0079] Casein phosphopeptide (purity not less than 85%) 117.65g
[0080] Taurine 30g
[0081] Xylitol 1500g
[0082] White sugar 1200g
[0083] Orange flavor 1g
[0084] The processing and precautions are as described in Embodiment 1.
Embodiment approach 3
[0085] Embodiment 3: The chewable tablet for discharging lead of the present invention includes
[0086] Glutathione (purity not less than 98%) 306g
[0087] Bovine colostrum powder (including 1000 grams of immunoglobulin) 2500g
[0088] Pearl powder 3000g
[0089] Vitamin C 1197g
[0090] Zinc gluconate (50 grams of zinc) 384.91g
[0091] Casein phosphopeptide (purity not less than 85%) 235.29g
[0092] Taurine 100g
[0093] Xylitol 2500g
[0094] White sugar 2000g
[0095] Orange flavor 5g
[0096] The processing and precautions are as described in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com