Novel synthesis process of anti-cancer Sunpla
A new process, anti-cancer drug technology, applied in the field of biopharmaceuticals, can solve the problems of long processing process, introduction of silver impurities, affecting product quality, etc., and achieve the effect of short synthesis route, improved product quality, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
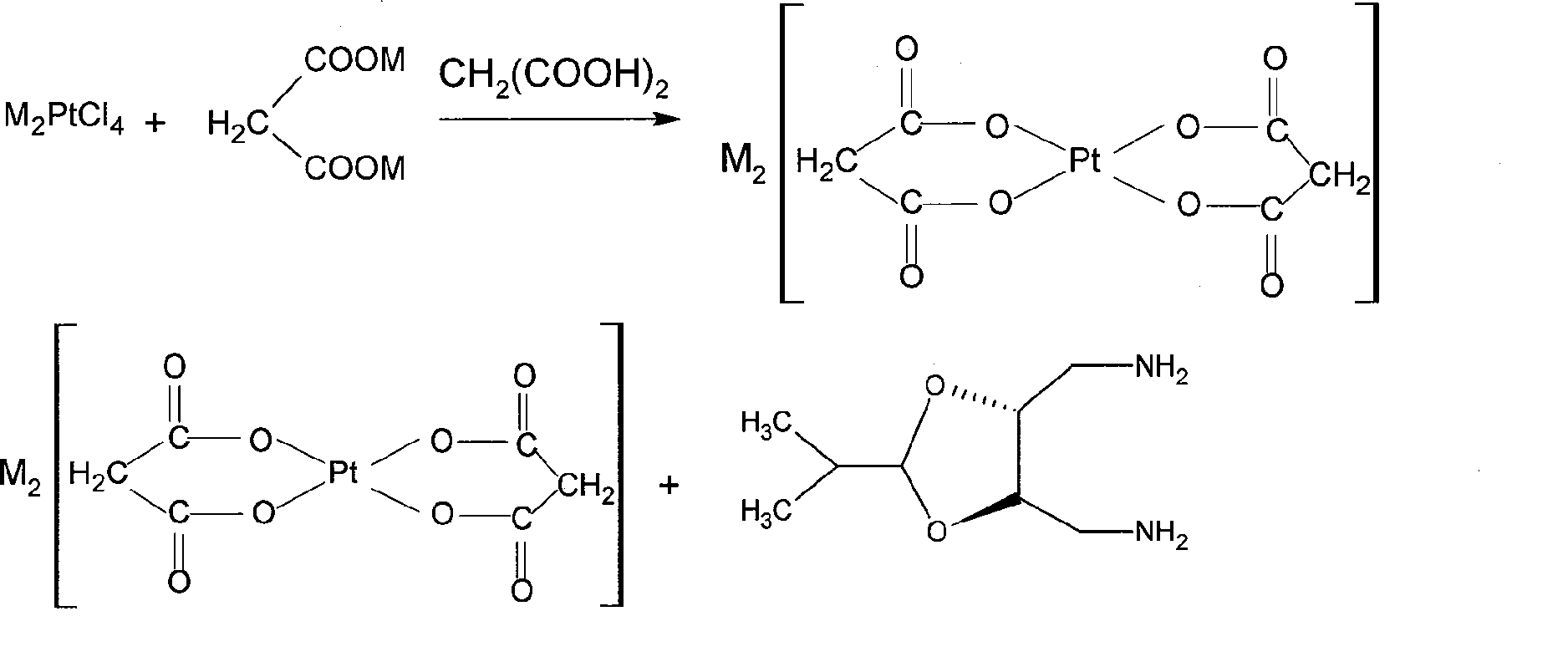
[0017] (1), the preparation of two (malonate) platinum (II) potassium
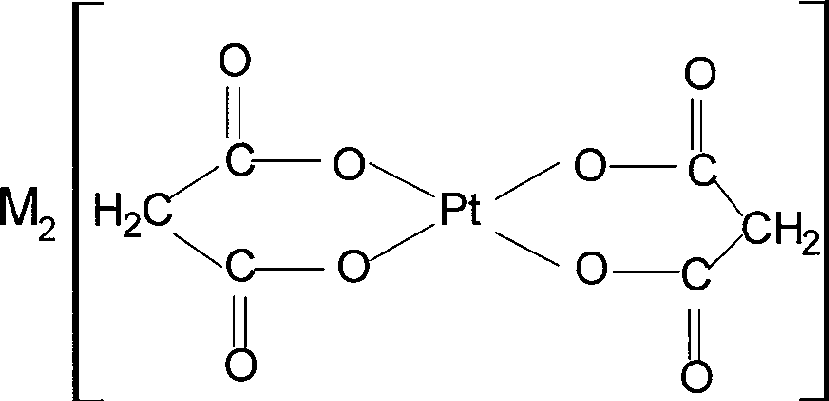
[0018] Take 10g (24mmol) K 2 PtCl 4 , dissolved in 100ml of water, added 35g (192mmol) CH 2 (COOK) 2 and 4.9g (48mmol) CH 2 (COOH) 2 , reacted at 75°C for 8 hours, cooled, precipitated yellow crystals, collected by filtration, washed with water and ethanol, and dried in vacuum at 65°C for 4 hours to obtain 9.63g K 2 {Pt[CH 2 (COO) 2 ] 2} 2H 2 O, 85% yield.
[0019] The characteristic structural parameters are: . Elemental analysis C 14.11%; H 1.57%; Pt 38.2%, consistent with the theoretical value C 14.04%; H 1.560%; Pt 38.0%. .FAB + -MS m / e=400 (Pt[CH 2 (COO) 2 ] 2+ , 45%). .IR(cm -1 , KBr tablet) 3572-3495 (s, vO-H, H 2 O) 2861 (w, vCH2), 1635 [vs vas (COO)], 1370 [svs (COO)]. These parameters conform to K 2 {Pt[CH 2 (COO) 2 ] 2} 2H 2 The chemical structure of O.
[0020] (2), the synthesis of Platinum
[0021] Take 4g (7.8mmol) K 2 {Pt[CH 2 (COO) 2 ] 2} 2H 2 O, dissolved in ...
Embodiment 2
[0028] (1), the preparation of two (malonate) platinum (II) sodium
[0029] Take 9.2g (24mmol) Na 2 PtCl 4 , dissolved in 100ml of water, added 28.4g (192mmol) CH 2 (COONa) 2 and 4.9g (48mmol) CH 2 (COOH) 2 , reacted at 75°C for 8 hours, cooled, precipitated pale yellow crystals, collected by filtration, washed with water and ethanol, and dried in vacuum at 65°C for 4 hours to obtain 7.5g Na 2 {Pt[CH 2 (COO) 2 ] 2} H 2 O, yield 75%, Pt42.8%.
[0030] (2), the synthesis of Platinum
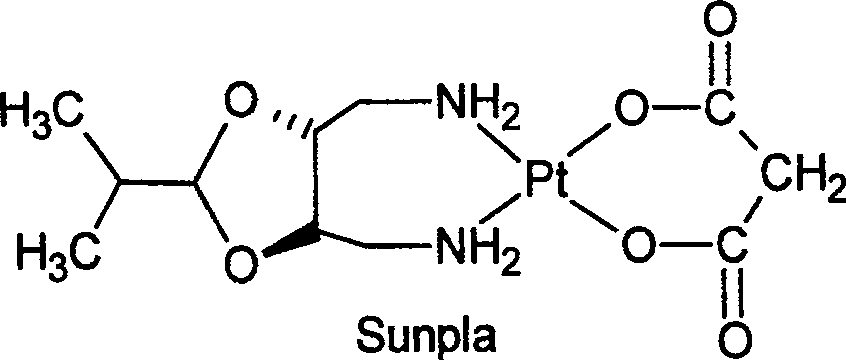
[0031] Take 3.55g (7.8mmol) Na 2 {Pt[CH 2 (COO) 2 ] 2} 2H 2 O, dissolved in 20ml of water, slowly add 1.6g (9.2mmol) of (4R,5R)-4,5-bis(aminomethyl)-2-isopropyl-1,3-dioxo After cyclopentane, it was heated to reflux for 12 hours, cooled, off-white crystals were precipitated, collected by filtration, washed with water and ethanol, and then vacuum-dried at 65° C. for 4 hours to obtain 2.34 g of splatin, with a yield of 65%.
[0032] (3), the purification of Shuplatin
[0033] Take 2g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com