Method for preparing medicinal accessories sodium caprylate
A pharmaceutical excipient, sodium caprylate technology, applied in carboxylate preparation, organic chemistry, etc., can solve problems such as poor solubility, large particles, and poor clarity, and achieve simple process, high quality, and protection from The effect of pyrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
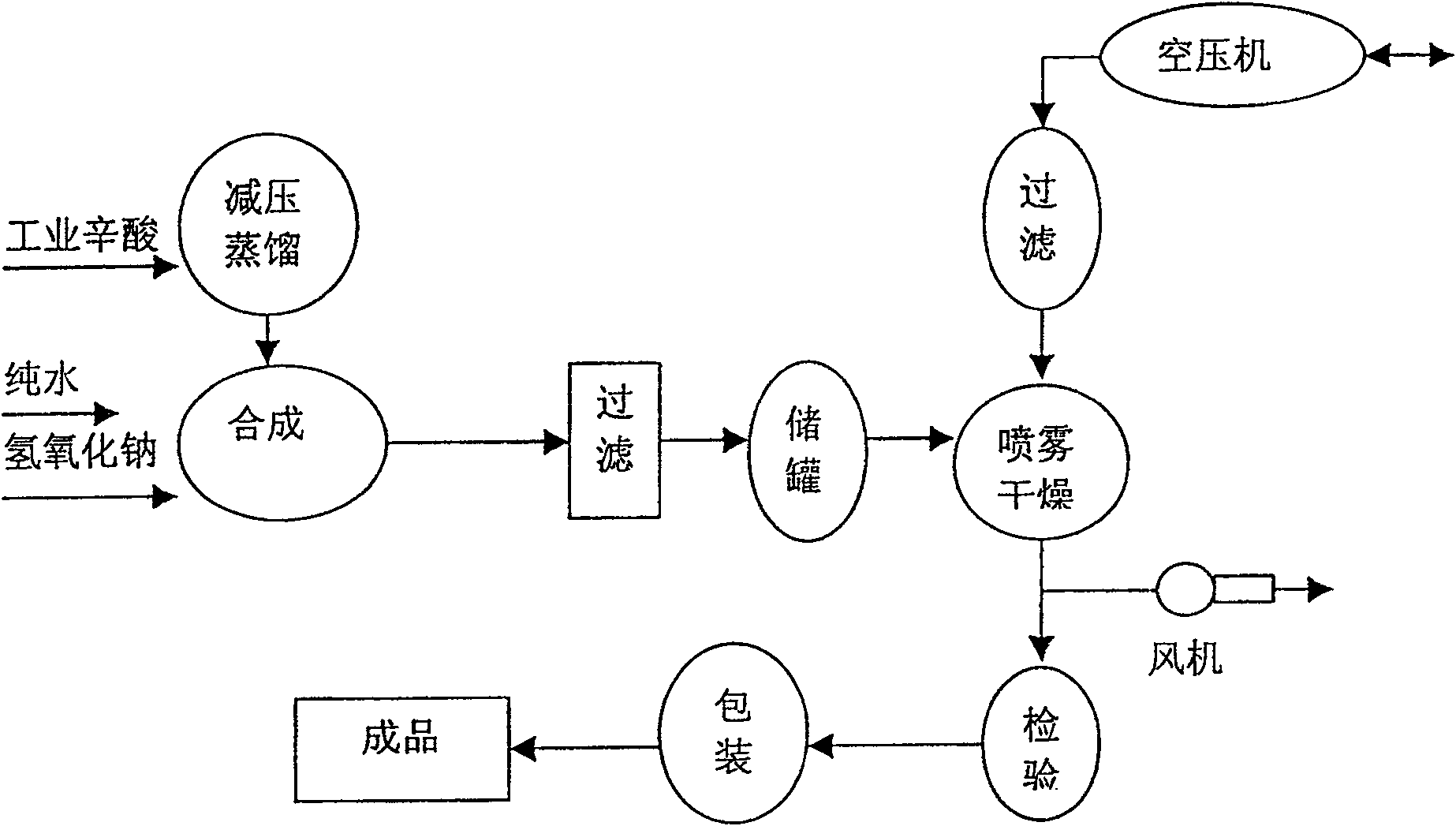
[0044] The preparation method of pharmaceutical adjuvant sodium caprylate described in the present embodiment, it may further comprise the steps:
[0045] ①Distillation purification and purification of octanoic acid:
[0046] Put the industrial octanoic acid into the acid distillation tank, use a vacuum pump to reduce the pressure in the system to 0.07Mpa, and carry out distillation at a temperature of 135°C. After the acid vapor enters the condenser to cool, the purified octanoic acid is separated and set aside;
[0047] ② Synthesis of sodium octanoate:
[0048] Dissolve 20Kg of sodium hydroxide in 140L of pure water. After completely dissolving, add 72Kg of purified caprylic acid while stirring. Heat the temperature to 80°C. After full reaction, sodium caprylate will be generated, and the pH value will be controlled at 8.5;
[0049] ③Spray drying:
[0050] After the sodium caprylate solution was allowed to stand, it was filtered to remove trace impurities, and then centrif...
Embodiment 2
[0058] The preparation method of pharmaceutical adjuvant sodium caprylate described in the present embodiment, it may further comprise the steps:
[0059] ①Distillation purification and purification of octanoic acid:
[0060] Put the industrial octanoic acid into the acid distillation tank, reduce the pressure in the system to 0.08Mpa with a vacuum pump, and carry out distillation at a temperature of 145°C. After the acid vapor enters the condenser to cool, the purified octanoic acid is separated and set aside;
[0061] ② Synthesis of sodium octanoate:
[0062] Dissolve 30Kg of sodium hydroxide in 180L of pure water. After completely dissolving, add 108Kg of purified caprylic acid while stirring. Heat the temperature to 100°C. After full reaction, sodium caprylate will be generated, and the pH value will be controlled at 9.5;
[0063] ③Spray drying:
[0064] After the sodium caprylate solution was allowed to stand, it was filtered to remove trace impurities, and then it was ce...
Embodiment 3
[0072] The preparation method of pharmaceutical adjuvant sodium caprylate described in the present embodiment, it may further comprise the steps:
[0073] ①Distillation purification and purification of octanoic acid:
[0074] Put the industrial octanoic acid into the acid distillation tank, reduce the pressure in the system to 0.10Mpa with a vacuum pump, and carry out distillation at a temperature of 150°C. After the acid vapor enters the condenser to cool down, separate the purified octanoic acid for later use;
[0075] ② Synthesis of sodium octanoate:
[0076] Dissolve 15Kg of sodium hydroxide in 130L of pure water. After completely dissolving, add 54Kg of purified caprylic acid while stirring, and heat the temperature to 110°C. After full reaction, sodium caprylate will be generated, and the pH value will be controlled at 10;
[0077] ③Spray drying:
[0078] The sodium octanoate solution was left to stand and then filtered to remove trace impurities, then centrifugally sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com