Bacterial compositions for the treatment of cancer
A cancer and tissue technology, applied in the direction of bacterial medical raw materials, drug combinations, bacterial antigen components, etc., can solve problems such as expensive, time-consuming, and limited use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
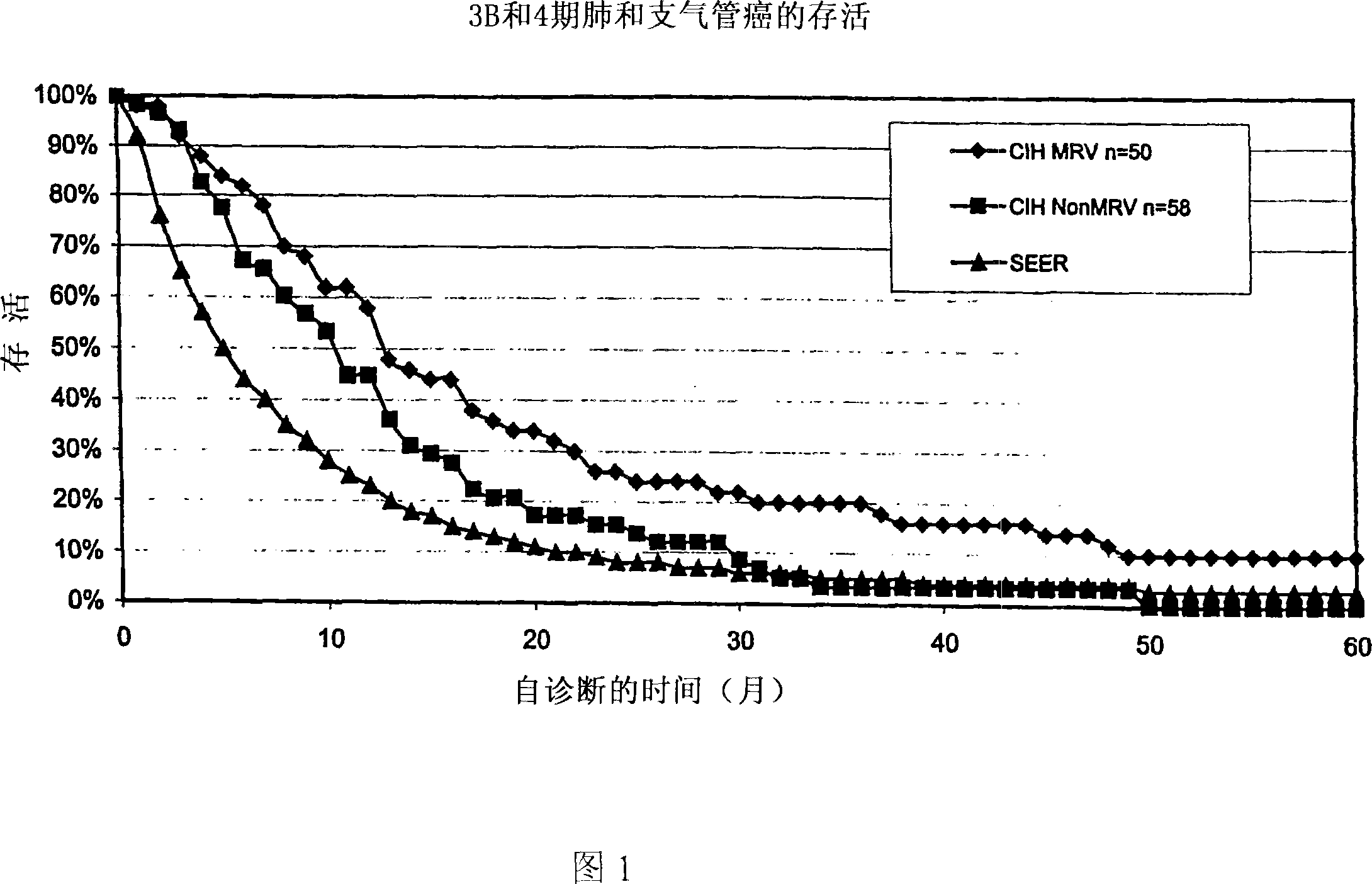
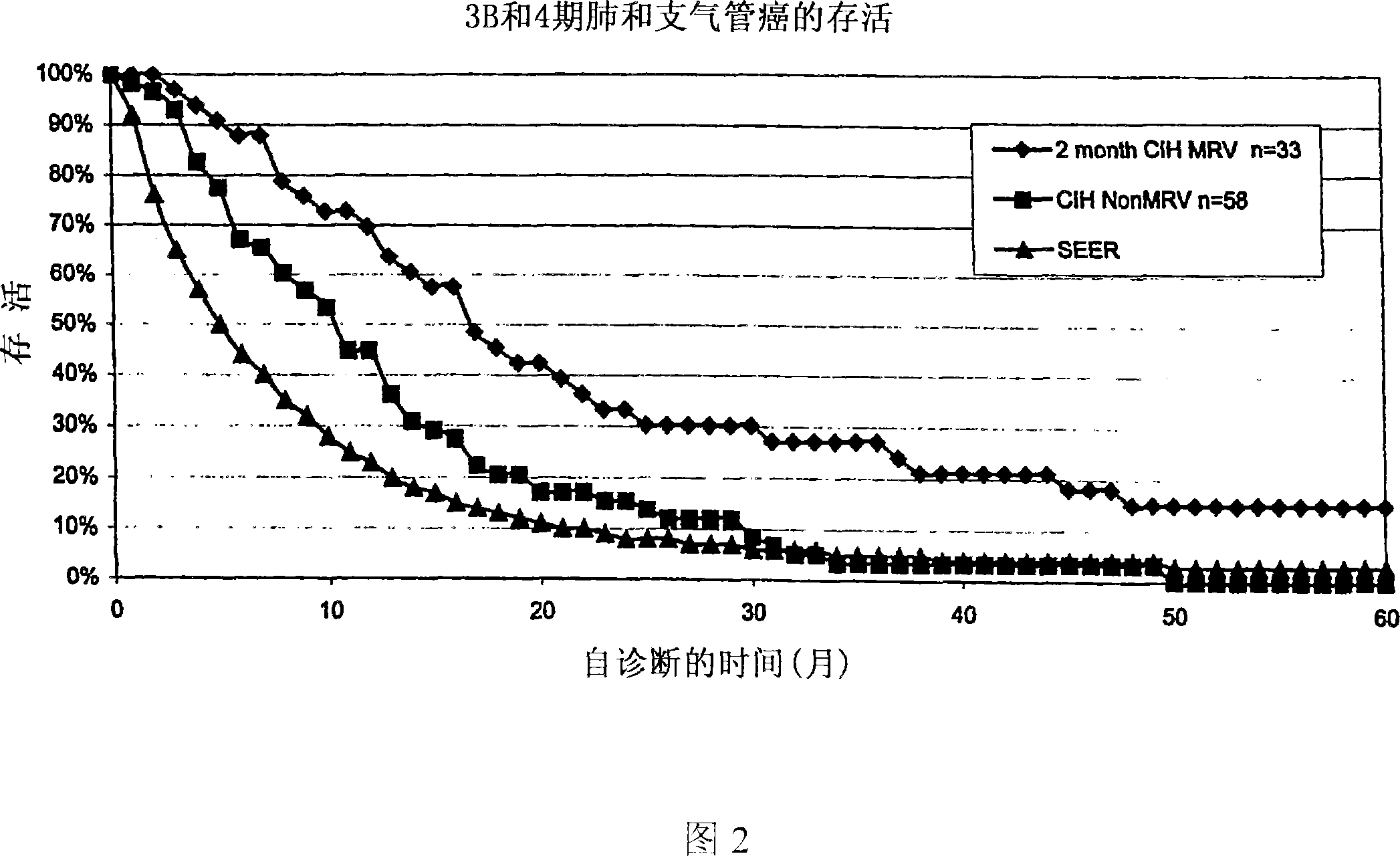
[0095] bacterial composition
[0096] Approximately 750 cancer patients of all cancer types and stages were treated with the mixed bacterial vaccine in a blinded study in which the patients were unaware of the composition of the vaccine. The following three mixed bacterial vaccines were used:
[0097] 1. Bayer Corporation MRV TM "Bayer MRV" (Hollister-Steir Laboratories, Spokane, WA, USA), including the following strains:
[0098] organism per ml
[0099] Staphylococcus aureus 1.2 billion
[0100] Viridans and non-hemolytic streptococci 200 million
[0101] Streptococcus pneumoniae 150 million
[0102] Moraxella catarrhalis (Neisseria) 150 million
[0103] Klebsiella pneumoniae 150 million
[0104] Haemophilus influenzae 150 million
[0105] The vaccine is prepared for the following indications: rhinitis, infectious asthma, chronic sinusitis, nasal polyposis and chronic serous otitis media. Cancer treatment is not indicated as an intended u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com