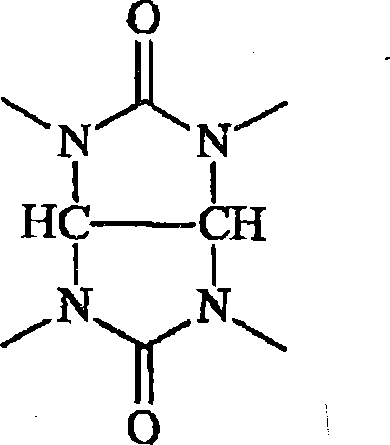
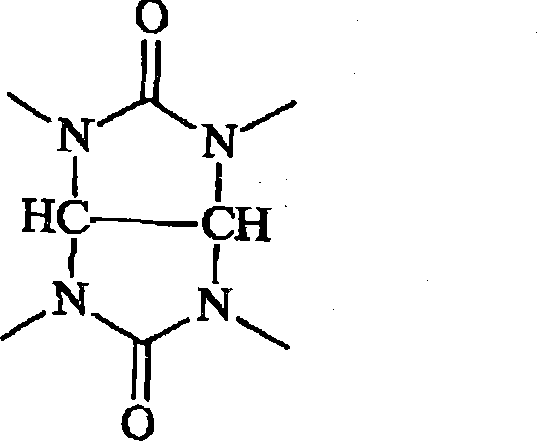
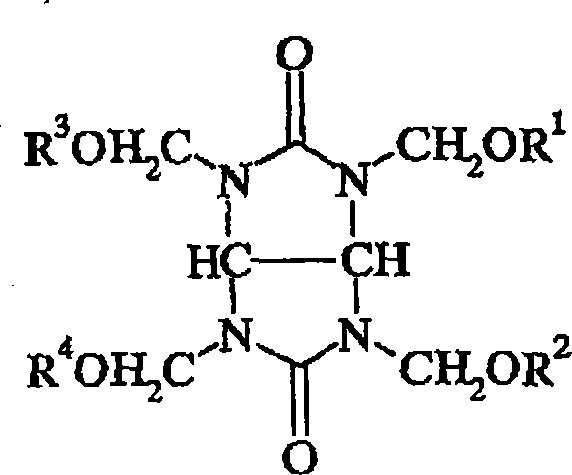
Antireflective compositions for photoresists
A photoresist, reactive compound technology, applied in the field of coating compositions, can solve the problems of ineffective imaging and etching, damage to photoresist patterns, inability to accurately transfer substrates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Charge 600 g of tetramethoxymethyl glycoluril, 96 g of styrene glycol, and 1200 g of propylene glycol monomethyl ether acetate (PGMEA) into a 2-liter (1) jacketed flask equipped with a thermometer, mechanical stirrer, and cold water condenser and heated to 85°C. A catalytic level of p-toluenesulfonic acid monohydrate was added and the reaction was maintained at this temperature for 5 hours. The reaction solution was then cooled to room temperature and filtered. The filtrate was slowly poured into distilled water to precipitate out the polymer. The polymer was filtered, rinsed thoroughly with water and dried in a vacuum oven (yielding 250 g of polymer). The resulting polymer had a weight average molecular weight of about 17,345 g / mol and a polydispersity of 2.7. h 1 NMR indicated that the polymer was a condensation product of two starting materials. The broad peak centered at 7.3 ppm is characteristic of the benzene moiety present in the polymer, and the broad peak ...
Embodiment 2
[0058] 260 g tetramethoxymethyl glycoluril, 41.6 g neopentyl glycol and 520 g PGMEA were charged into a 21 jacketed flask with thermometer, mechanical stirrer and cold water condenser and heated to 85°C. A catalytic level of p-toluenesulfonic acid monohydrate was added and the reaction was maintained at this temperature for 5 hours. The reaction solution was then cooled to room temperature and filtered. The filtrate was slowly poured into distilled water while stirring to precipitate out the polymer. The polymer was filtered, rinsed thoroughly with water and dried in a vacuum oven (yielding 250 g of polymer). The resulting polymer had a weight average molecular weight of about 18,300 g / mol and a polydispersity of 2.8. The broad peak centered at 0.9 ppm is attributed to the methyl group of neopentyl glycol, and the broad peak centered at 3.3 ppm is the unreacted methoxy group on tetramethoxymethyl glycoluril (CH 3 O), which indicates that the resulting polymer is a condensat...
Embodiment 3
[0060] 50 g of tetramethoxymethyl glycoluril, 23.9 g of styrene diol and 35 g of 2-methyl-2-nitropropanediol were charged into a 500 ml jacketed flask equipped with a thermometer and a mechanical stirrer. The reaction mixture was heated to 100 °C to obtain a clear solution. A catalytic level of p-toluenesulfonic acid monohydrate was added and the reaction was held for 60 minutes. Then 60 g of PGMEA was added and the reaction was held for a further 2 hours. The reaction solution was then cooled to room temperature and filtered. The filtrate was slowly poured into ether with stirring to precipitate the polymer. The polymer was filtered, rinsed thoroughly with ether and dried in a vacuum oven (yield 33 g of polymer). The resulting polymer had a weight average molecular weight of about 6,305 g / mol and a polydispersity of 2.6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com