Aspartic acid laumosaren hydrate, its production and use
A technology of lomefloxacin aspartate and hydrate, which is applied in the field of medicine and can solve problems such as the preparation method and application of lomefloxacin aspartate hydrate that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
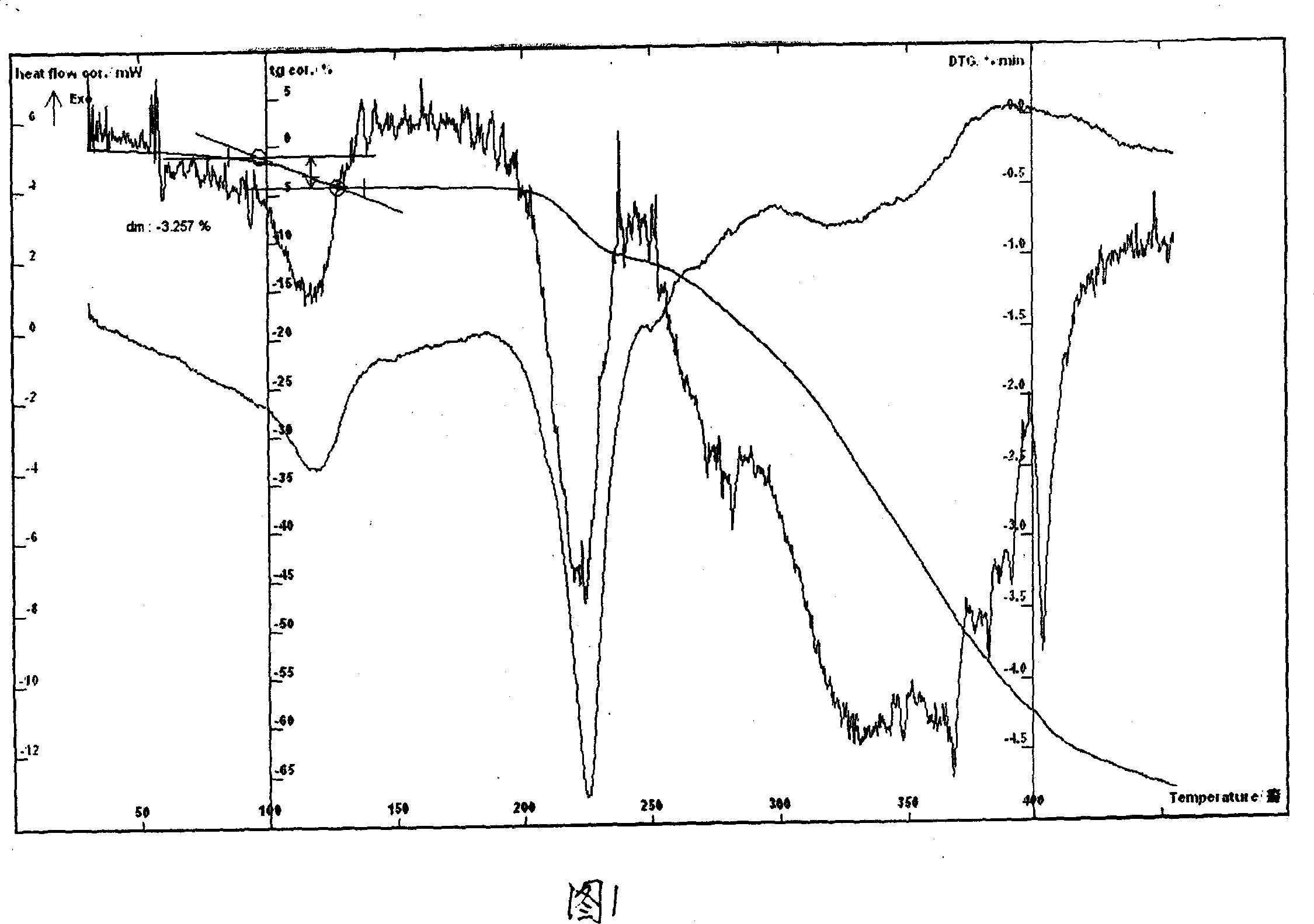
[0029] Example 1 In a three-necked flask, add 17.57g of lomefloxacin to 100ml of 95% ethanol, heat to reflux to dissolve, add 20ml of an aqueous solution of 6.66g of L-aspartic acid, stir at 40-100°C until reflux for 2h, and react After completion, slowly add 180ml of absolute ethanol, cool to below 0°C, wait for the solid to precipitate, filter, rinse the solid with ethanol several times, drain it, and dry it at 78°C for 6 hours to obtain 20.56g of off-white crystalline powder, MS ( ESI, EI) m / e 483 (M-18), 351 (M-133). HPLC analysis: purity 99.5%, the retention time of the main peak of the sample is consistent with that of the main peak of commercially available lomefloxacin aspartate small water needle. The water content determined by the Karl Fischer method is 3.69%, which is consistent with the result that the sample contains 1 crystal water (theoretical value 3.58%). Thermal analysis test (TG-DTG): The sample lost about 3.26% of its weight at 80-140°C. The thermal analys...
Embodiment 2
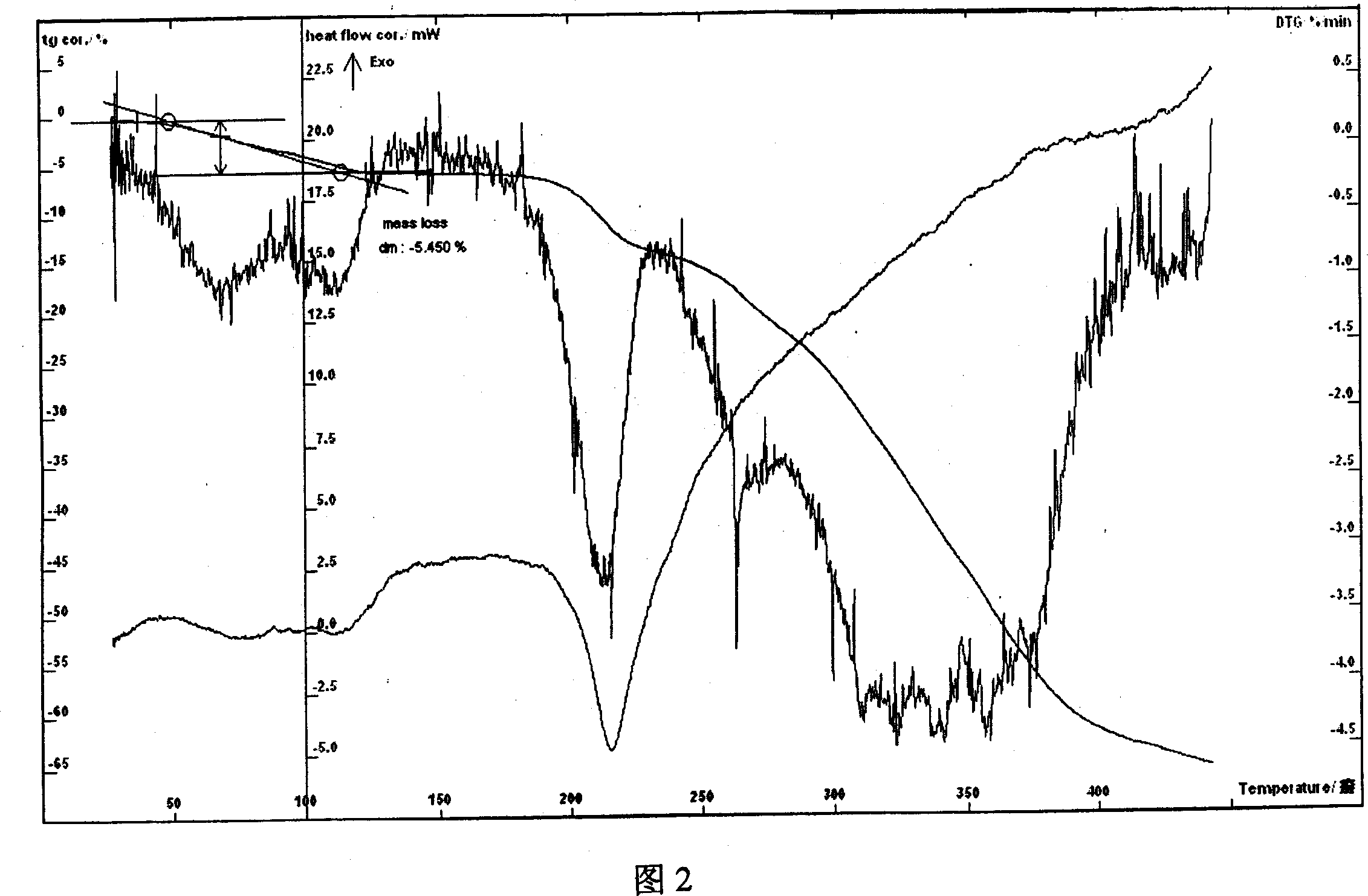
[0032] Example 2 In a three-necked flask, add 10.21g of lomefloxacin and 3.87g of aspartic acid, add 10ml of water and 60ml of ethanol, heat to dissolve, stir continuously at 70-90°C to complete the reaction, slowly add 120ml of absolute ethanol, cool To about 0°C, wait for the solid to precipitate, filter, rinse the solid with ethanol, drain, and dry at 80°C for 4 hours to obtain 10.82g of off-white powder. The retention time of the main peak of methfloxacin small water needle is the same. Moisture (Karl Fischer method): 3.76%, thermal analysis test (TG-DTA) shows: the weight loss of the sample is not obvious before 70°C, and the weight loss is about 3.36% at 80-130°C, which is consistent with the result that the sample contains 1 crystal water (theoretical Value 3.58%) consistent, thermal analysis test shows that there is an obvious endothermic peak between 80 ~ 130 ℃, indicating that the sample contains water of crystallization, the measured value and the theoretical value ...
Embodiment 3
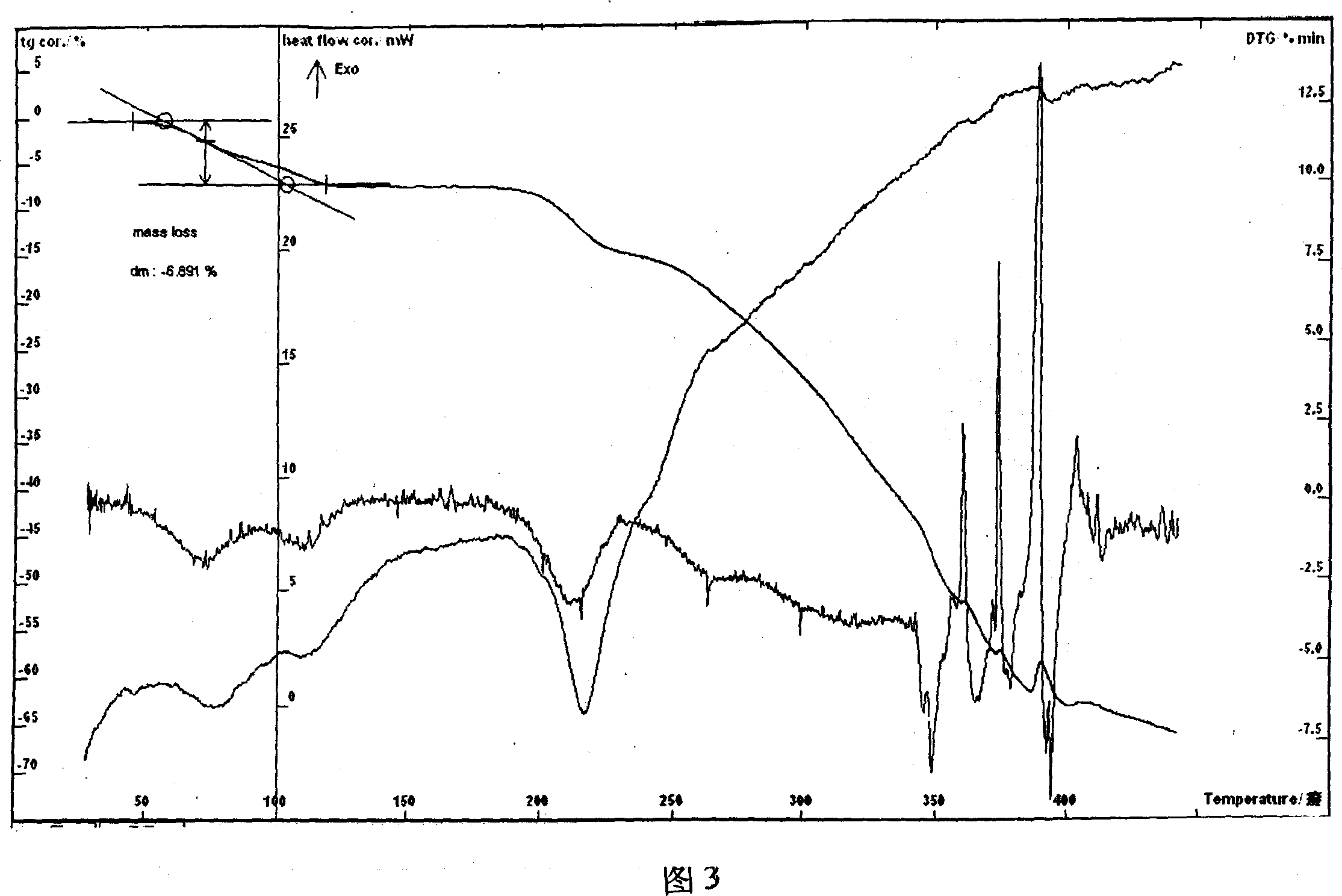
[0035] Example 3 Put 9.28g of lomefloxacin and 3.52g of L-aspartic acid into a reaction flask, add 60ml of water, heat to reflux, then control the temperature between 50-80°C, stir and react for 2h, after the reaction is completed, put it Freeze-dry, crystallize the obtained solid with about 22 times the volume of water, ethanol and isopropanol mixed solvent (2:10:10), cool, filter at low temperature, rinse with absolute ethanol, drain, and dry at 50°C for 8 hours Obtained 9.76 g of L-aspartic acid lomefloxacin dihydrate. HPLC analysis: purity 99.4%, the retention time of main peak of sample and commercially available aspartic acid lomefloxacin small water needle main peak is consistent, and Karl Fischer's method measures water and is 6.79%, and this and sample contain the result of 2 crystal waters (theory Value 6.92%) consistent, thermal analysis test (TG-DTG): sample, 50 ~ 140 ° C weight loss of about 6.89%, thermal analysis test (TG-DTA see Figure 3) shows that there is an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com