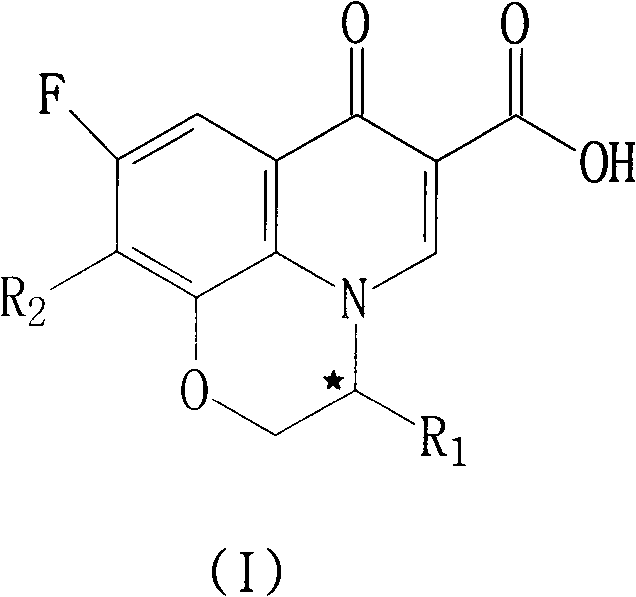
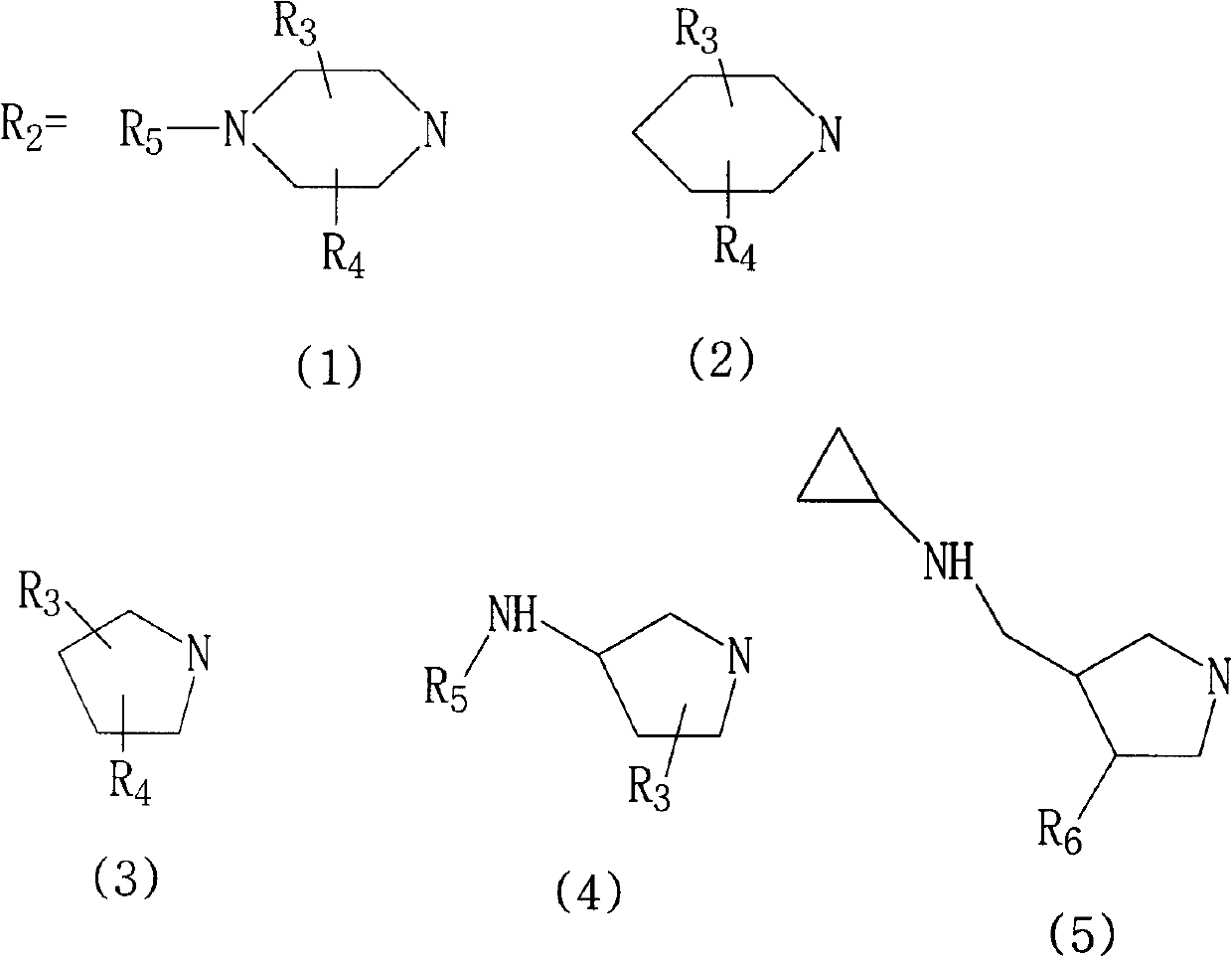
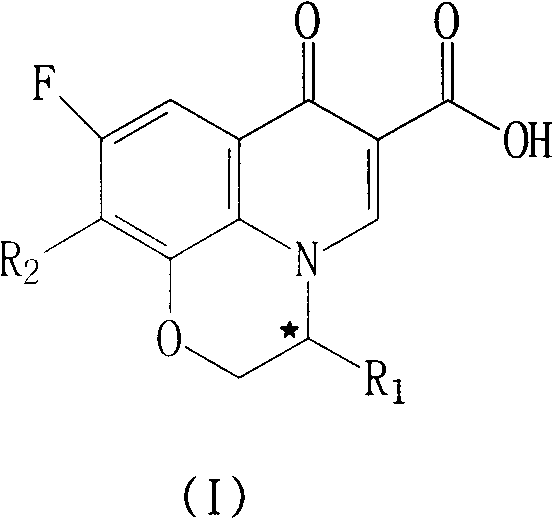
Fluoroquinolone compounds and synthesis method thereof
A technology of fluoroquinolones and compounds, which is applied in the field of preparation of fluoroquinolones and can solve urgent problems such as
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0023] Example 1a: Synthesis of (Z)-3-[[2,2-difluoro-1-(hydroxymethyl)ethyl]amino]-2-(2,3,4,5-tetrafluorobenzoyl)propane -Ethyl 2-enoate (3a)
[0024] Ethyl 2,3,4,5-tetrafluorobenzoylacetate (1) (100 g, 0.378 mol), triethoxyorthoformate (63 mL, 0.567 mol), and acetic anhydride (89 ml, 0.945 mol) mixture was heated to 115-120°C and stirred for three hours, then concentrated in vacuo; the concentrate was diluted with 455ml of ethanol, and the diluted solution was cooled to zero in an ice bath, and the solution was added dropwise with (2S)-2-amino -3,3-Difluoropropanol (33.7 grams, 0.416mol) and 410ml ethanol generated solution; The resulting mixture was stirred at room temperature for one hour, then concentrated in vacuo; The concentrate was further purified on a silica gel column with dichloromethane / The methanol mixture was eluted to give 94 g of a pale yellow solid in a 62% yield.
[0025] 3a: 1 HNMR (CDCl 3 )δ: 1.26(t, J=6.9Hz, 3H), 3.13-3.36(m, 2H), 3.48-3.53(dd, J=12....
Embodiment 1b
[0026] Example 1b: Synthesis of (Z)-3-[[2,2-difluoro-1-(hydroxymethyl)ethyl]amino]-2-(2,3,4,5-tetrafluorobenzoyl)propane -2-enoic acid ethyl ester (3b)
[0027] The intermediate (2) was reacted with (2S)-2-amino-3,3,3-trifluoropropanol in the same way as in the synthesis of (3a) to obtain 26 g of the compound with a yield of 18%.
[0028] 3b: 1 HNMR (CDCl 3 )δ: 1.29(t, J=7.0Hz, 3H), 3.48-3.53(m, 2H), 3.86(dd, J=9.0, 7.0Hz, 1H), 4.20(q, J=7.0Hz, 2H), 8.20(m, 1H), 10.9(s, 1H); MS(ES + ): MW+H + = 404.1.
Embodiment 2a
[0029] Example 2a Synthesis of (3R)-9,10-difluoro-2,3-dihydro-3-difluoromethyl-7-oxidation-7hydropyrido[1,2,3-de][1,4 ]Benzoxazine-6-carboxylic acid ethyl ester (4a)
[0030] A mixture of intermediate (3a) (66 g, 0.172 mol), anhydrous potassium fluoride (spray-dried, 35 g, 0.60 mol) and anhydrous dimethyl sulfone (300 ml) was heated to 115-120 °C for five hours After cooling to room temperature, 150ml of methanol was added; the resulting mixture was stirred in an ice bath for two hours, then filtered; the collected precipitate was resuspended in 250ml of methanol and stirred for 30 minutes, and filtered; the collected precipitate was resuspended in 250 Stir in mL of water for 30 minutes, filter, wash with 125 mL of methanol, suck dry, and dry in vacuo to obtain 33 g of light yellow solid (4a); the yield is 56%.
[0031] 4a: 1 HNMR (CDCl 3)δ: 1.28(t, J=6.8Hz, 3H), 3.76(m, 1H), 4.03(dd, J=12.4, 7.2Hz, 1H), 4.18-4.30(m, 3H), 5.85(td, J =57.3, 7.0Hz, 1H), 7.63(dd, J=10.8, 7.8H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com