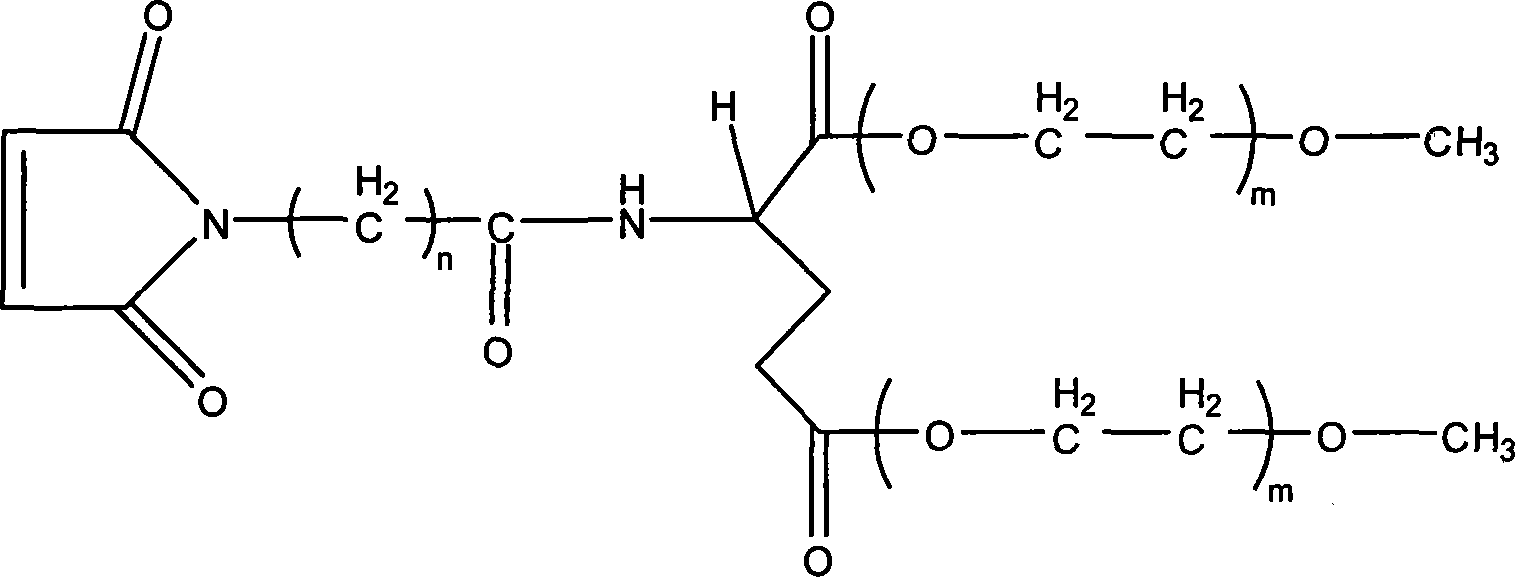
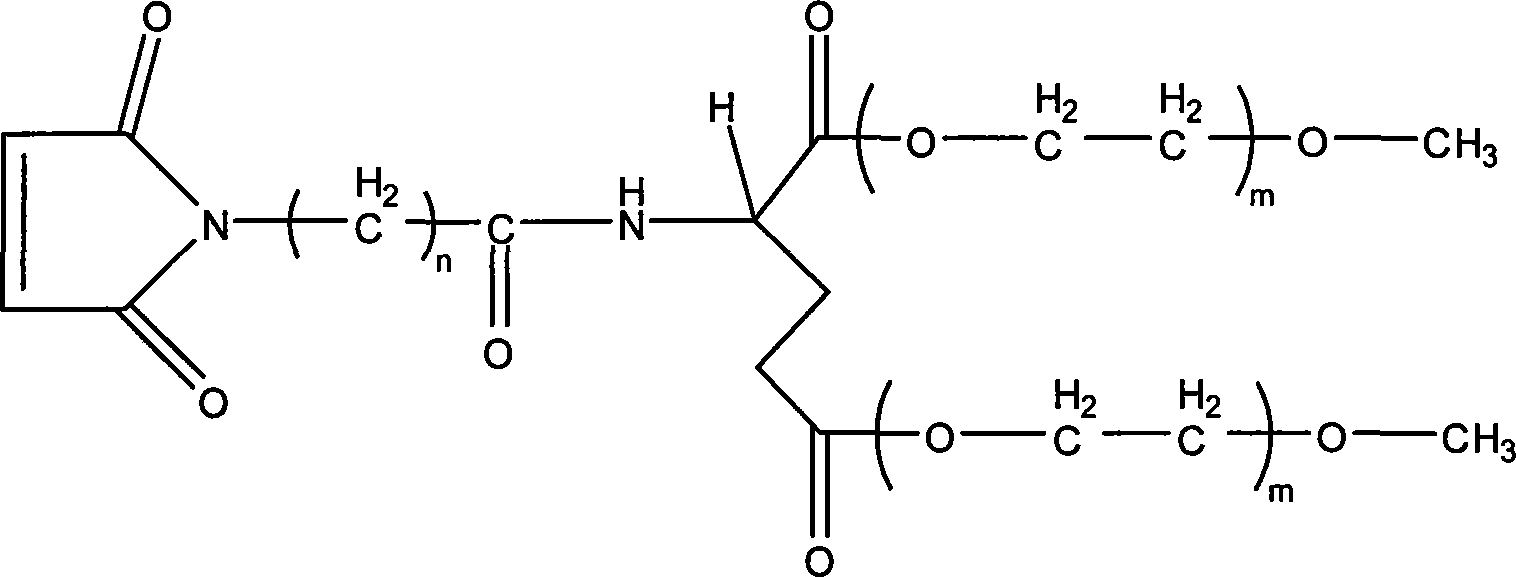
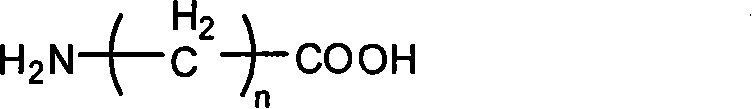Branching poly-ethylene and its production
A polyethylene glycol and branched technology, applied in the field of branched polyethylene glycol and its preparation, to achieve the effects of reducing the generation of isomers, reducing the loss of biological activity, and reducing the steric hindrance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1.Fmoc-Glu-(mPEG 750 ) 2 Preparation
[0030] Respectively 3.7 grams of Fmoc-Glu, 22.5 grams of monomethoxy polyethylene glycol (mPEG) with a molecular weight of 750 750 ) And 0.9 g of DMAP are placed in a long-necked flask, add 100 ml of analytically pure dichloromethane and 10 ml of analytically pure tetrahydrofuran, put in a magnet, stir at room temperature to dissolve the reactants, 10 minutes later, add 8.0 grams of DCC, The reaction was stirred at 0°C for 24 hours. After the reaction, the temperature control was stopped, and the temperature was raised to room temperature. The reaction was continued for 1 hour. After removing the white precipitate by filtration, 4.0 g of oxalic acid was added, and the reaction was stirred at room temperature for 1 hour. After filtration, the filtrate was concentrated to 20 by rotary evaporation at a temperature of 45°C. After 1 milliliter, it was naturally cooled to room temperature, 200 milliliters of anhydrous ether was added, and th...
Embodiment 2
[0039] 1.Fmoc-Glu-(mPEG 2000 ) 2 Preparation
[0040] Respectively, 4.1 grams of Fmoc-Glu and 60.0 grams of monomethoxy polyethylene glycol (mPEG) with a molecular weight of 2000 2000 ) And 0.8 g of DMAP are placed in a long-necked flask, add 150 ml of analytically pure dichloromethane and 15 ml of analytically pure tetrahydrofuran, put in a magnet, and stir at room temperature to dissolve the reactants. After 15 minutes, add 6.0 grams of DCC. The reaction was stirred at 5°C for 26 hours. After the completion of the reaction, the temperature control was stopped, the temperature was raised to room temperature naturally, and the reaction was continued with stirring for 1 hour. After the white precipitate was removed by filtration, 3.2 g of oxalic acid was added, the reaction was stirred at room temperature for 1 hour, filtered, and the filtrate was concentrated to 30 ml by rotary evaporation at a temperature of 45° C., and then naturally cooled to room temperature, 300 ml of anhydro...
Embodiment 3
[0049] 1.Fmoc-Glu-(mPEG 5000 ) 2 Preparation
[0050] Respectively 4.4 grams of Fmoc-Glu, 150.0 grams of monomethoxy polyethylene glycol (mPEG) with a molecular weight of 5000 5000 ) And 0.7 g of DMAP are placed in a long-necked flask, add 300 ml of analytically pure dichloromethane and 30 ml of analytically pure tetrahydrofuran, put in a magnet, and stir at room temperature to dissolve the reactants. After 20 minutes, add 7.2 grams of DCC. The reaction was stirred at -5°C for 30 hours. After the completion of the reaction, the temperature control was stopped, the temperature was raised to room temperature naturally, and the reaction was continued with stirring for 1 hour. After the white precipitate was removed by filtration, 3.5 g of oxalic acid was added, the reaction was stirred at room temperature for 1 hour, filtered, and the filtrate was concentrated to 50 ml by rotary evaporation at a temperature of 45°C, and then naturally cooled to room temperature, 500 ml of anhydrous e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com