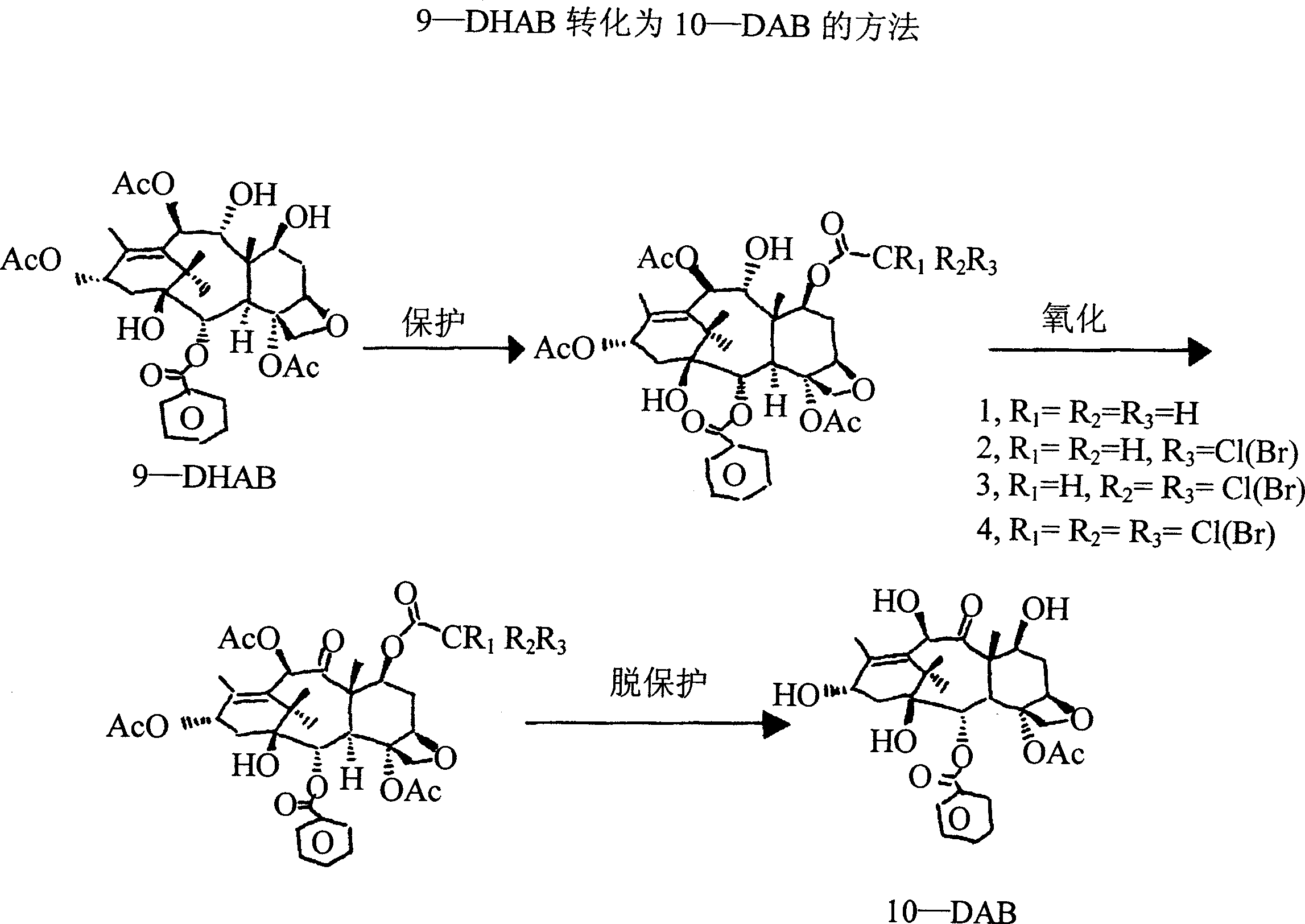
Conversion 9-dihydro-13-acetylbaccatin iii to 10-deacetylbaccatin iii
A technology of acetylbaccatin and deacetylbaccatin, applied in the direction of organic chemistry, etc., can solve the problem of low yield of the target product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0026] Conversion of 9-dihydro-13-acetylbaccatin III to 10-deacetylbaccatin III
[0027] 1. Purification of crude product 9-dihydro-13-acetylbaccatin III (9-DHA)
[0028] Put the crude 9-DHA (9-dihydro-13-acetylbaccatin III) into a round bottom flask, add 5-10 times methanol, and reflux the mixture for 1 hour or until all the 9-DHA is completely dissolved Afterwards, some methanol-insoluble yellow solid was removed by filtration. The supernatant was concentrated to remove most of the solvent and left at room temperature overnight. The resulting white crystals were filtered out, and the needle-shaped crystals were dried in an oven at 80-100° C. to obtain white needle-shaped 9-DHA with a purity greater than 98%.
[0029] 2. 9-DHA protection
[0030] Choose one of the methods
[0031] 2.1: 10g 9-DHA was dissolved in 100ml dichloromethane, stirred at room temperature for 5 minutes, then added 1.5mol tetrabutylammonium iodide and 5mol acetyl chloride, and the mixture was stirre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com