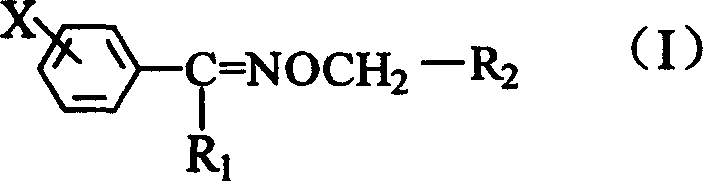Purification method of non-fatty oxime ether pyrethrin compound
A kind of technology of oxime etherethrin and purification method, applied in the directions of oxime preparation, organic chemistry, etc., can solve the problems of complicated process, low product yield, difficult to handle and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Purification of isopropyl-4-chlorophenone oxime-O-(3-phenoxybenzyl) ether
[0013] In a 500mL three-necked flask equipped with a stirring and thermometer, add 100g of industrial product of isopropyl-4-chlorophenoneoxime-O-(3-phenoxybenzyl)ether with a purity of 66.3%, petroleum ether: methanol : Methyl formate=9:6:0.9 mixed solvent 200mL, after stirring and mixing, put it in a low temperature bath, and cool for 8 hours at 0℃~-15℃. filter. The crystals were washed with cold petroleum ether, and dissolved under reduced pressure to obtain 65.85 g of a product with a purity of 95.6% and a yield of 82.5%.
Embodiment 2
[0015] Purification of 1-(4-chlorophenyl)-2-methylthio-O-[(3-phenoxyphenyl)methyl]acetone oxime ether 76.25% 1-(4-chlorophenyl)-2-methylthio-O-[(3-phenoxyphenyl)methyl]acetone oxime ether industrial product 115.7g, petroleum ether: absolute ethanol: ethyl acetate Ester=3:1:0.3 mixed solvent 230mL, after stirring evenly, put it in a low temperature bath, and cool for 24 hours at -10℃~-15℃. filter. The crystals were washed with cold petroleum ether, and dissolved under reduced pressure to obtain 76.4 g of a product with a purity of 96.1% and a yield of 83.22%.
Embodiment 3
[0017] Purification of 5-chloro-3-methyl-1-phenylpyrazole-4-carbaldehyde oxime-O-2-chloro-5-pyridyl methyl ether
[0018] In a 500 mL three-neck flask equipped with a stirring and thermometer, add 100 g of the above title compound with a purity of 65.1%, 200 mL of petroleum ether: isopropanol: isobutyl acetate = 6: 3:1 mixed solvent, and after stirring, put it in Cool in a low temperature bath at -15℃~-25℃ for 16 hours. filter. The crystals were washed with cold petroleum ether, and dissolved under reduced pressure to obtain 64.7 g of a product with a purity of 95.3% and a yield of 82.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com