Purine and pyrimidine cdk inhibitors and their use for the treatment of autoimmune diseases
An autoimmune and inhibitory technology, which is applied in the field of CDK2 and/or CDK7 and/or CDK9 inhibitors to treat diseases related to antinuclear antibodies, and can solve side effects and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
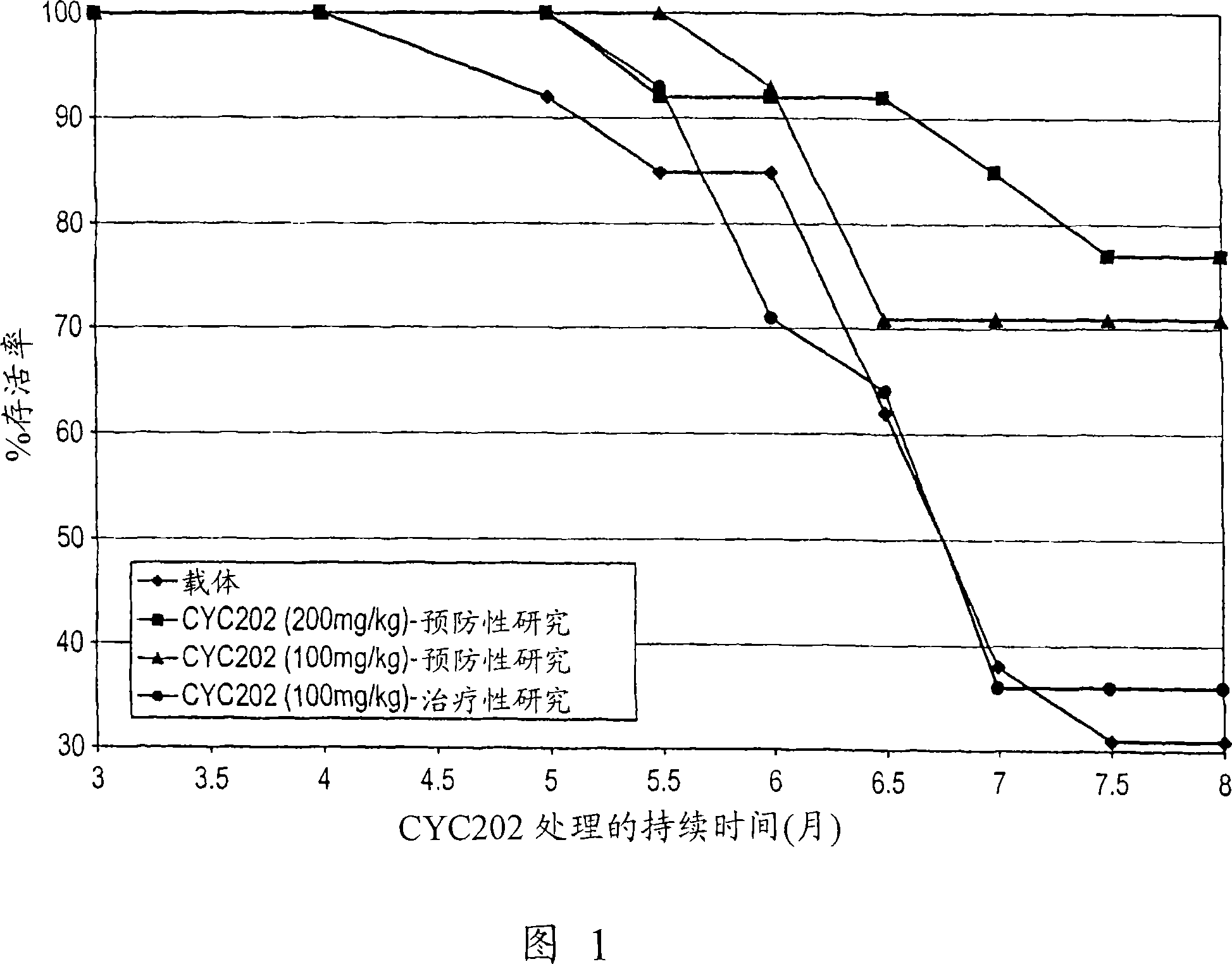
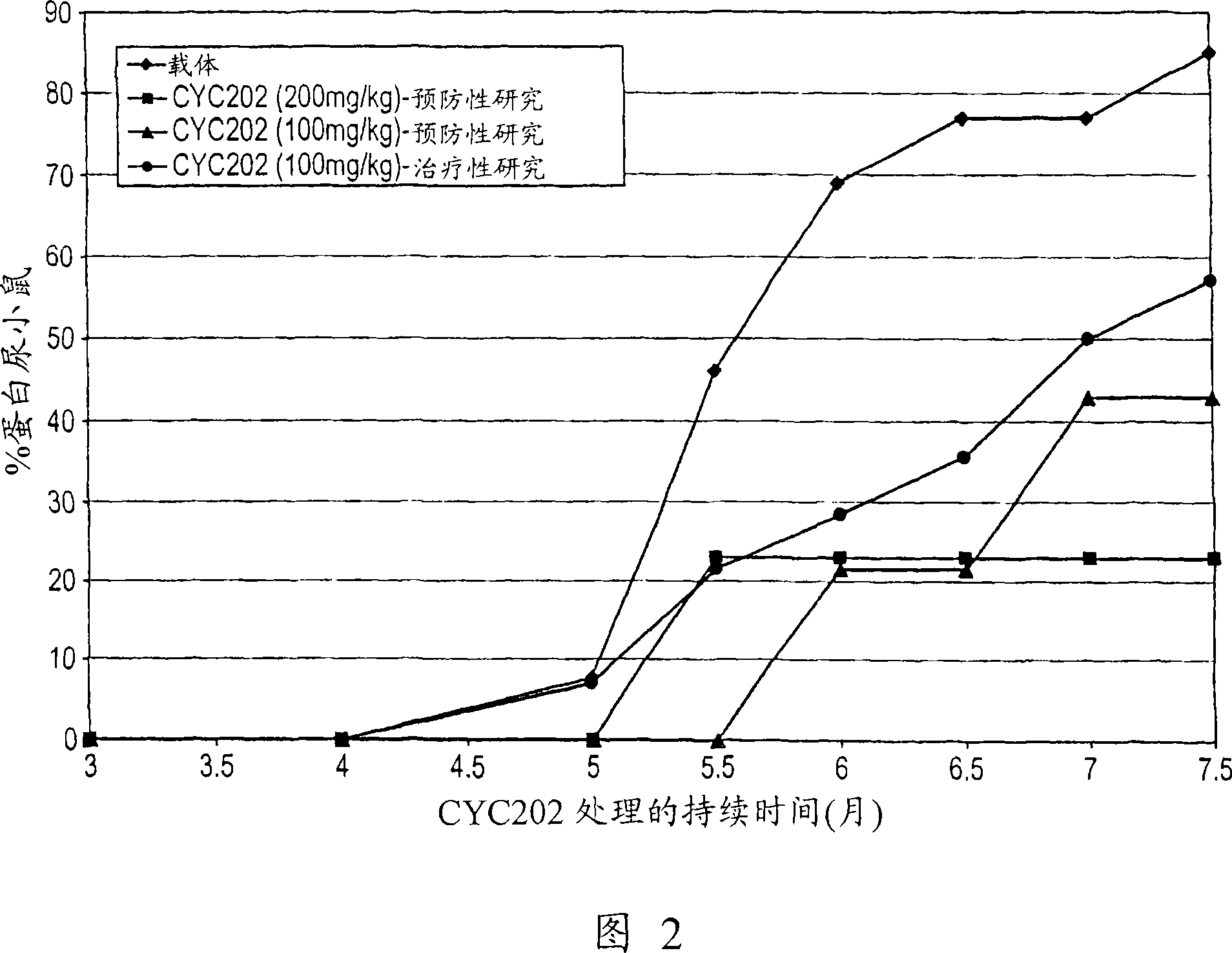
[0190] NZBxNZW F1 mice were randomly divided into the following groups:
[0191] Group 1 (n=18): mice gavaged with vehicle (HCl 50 mM) daily;
[0192] Group 2 (n=17): mice fed with CYC202 (200 mg / kg) daily;
[0193] Group 3 (n=19): Mice fed with CYC202 (100 mg / kg) daily;
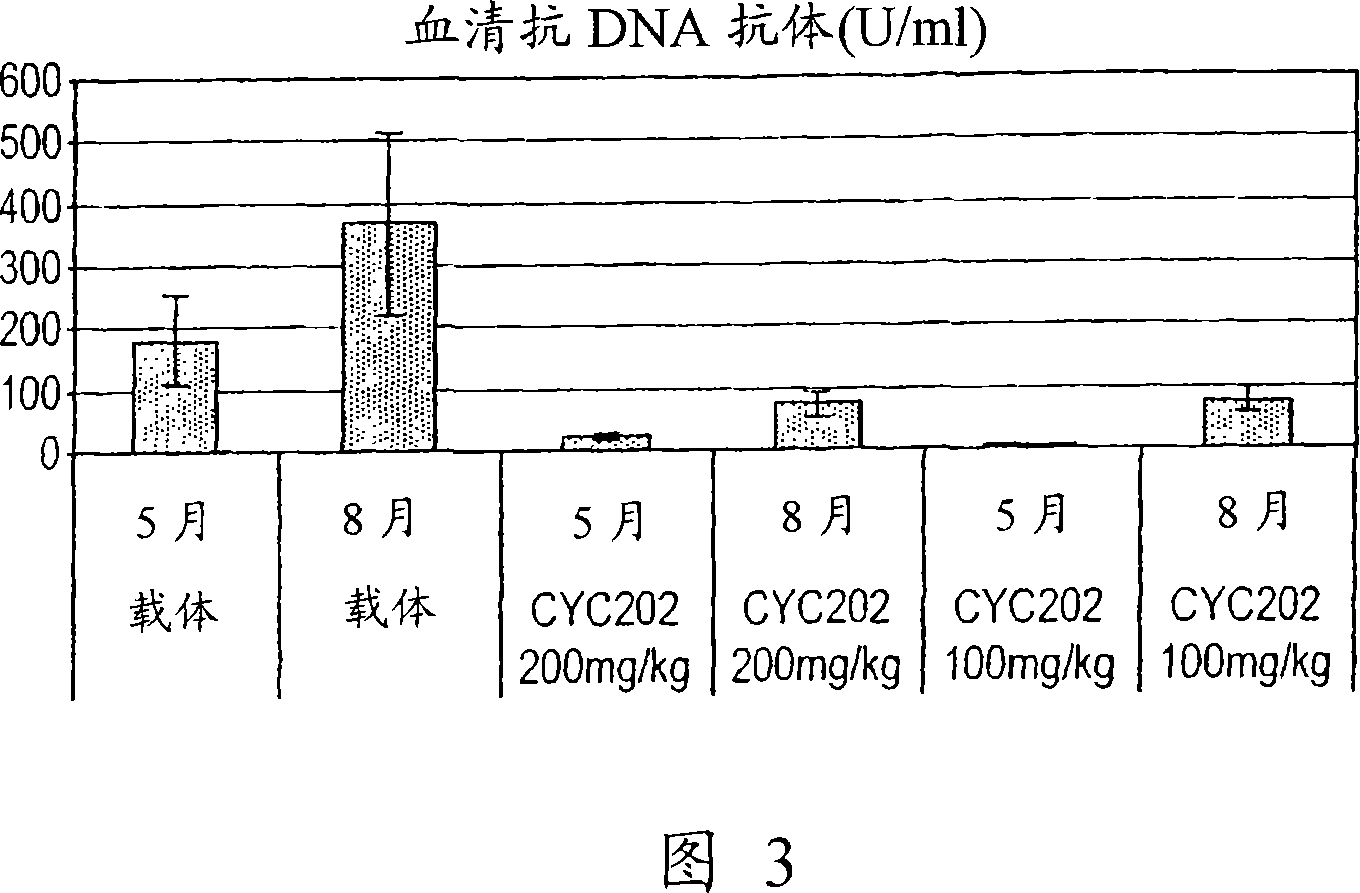
[0194] Treatment started at 2 months of age (prevention study) and continued until 8 months of age; at 5 months of age, 4-5 animals per group were sacrificed to assess levels of serum BUN and circulating anti-DNA antibodies.
[0195] Another group, group 4 (n=14 mice), was gavaged daily with CYC202 (100 mg / kg) starting at 5 months of age and continued until 8 months of age, at which point immune complex precipitation had actually occurred ( treatment research). Five normal CD-1 mice (Charles River Italia, Calco, Italy) served as controls.
[0196] The following parameters are evaluated:
[0197] Urinary protein excretion: measured monthly until 5 months of age, then every 2 weeks.
[0198] At execution...
Embodiment 2
[0205] Lupus mice were randomly divided into the following groups:
[0206] Group 1 (n=10): mice gavaged with vehicle (HCl 50 mM) daily;
[0207] Group 2 (n=15): Mice fed with CYC202 daily at a dose of 200 mg / kg;
[0208] Group 3 (n=12): Mice were injected intraperitoneally with methylprednisolone (MPS, Urbason, Hoechst s.p.a, Milano, Italy) daily at a dose of 1.5 mg / kg;
[0209] Group 4 (n=16): mice dosed daily with CYC202 (200 mg / kg) in combination with MPS (1.5 mg / kg).
[0210] Treatment started at 5 months of age, when immune complex precipitation had actually occurred, and continued until 12 months of age, when the last animal to receive vehicle treatment died. Five normal CD-1 mice (Charles River Italia, Calco, Italy) served as controls.
[0211] The following parameters are evaluated:
[0212] Survival rate;
[0213] Urinary protein excretion: measured monthly until 5 months of age, then every 2 weeks.
[0214] Serum BUN: measured at 5 months of age (before treatm...
Embodiment 1 and 2
[0217] Examples 1 and 2: Materials and methods
[0218] Proteinuria and Renal Function
[0219] Proteinuria concentrations were determined by the Coomassie Brilliant Blue G dye-binding assay with bovine serum albumin as the standard. Renal function was evaluated by BUN in heparinized blood by Reflotron test (Roche Diagnostics corporation, Indianapolis, USA). BUN levels above 30 mg / dl are considered abnormal (the normal range for mice is considered by this laboratory to be 14-29 mg / dl).
[0221] Levels of autoantibodies against dsDNA in serum were assessed by an enzyme immunoassay as previously described (Kidney Int, 53:726-734, 1998) (Diastat anti-dsDNA kit, Bouty Laboratory, Milano, Italy).
[0222] serum transaminase
[0223] Serum levels of AST and ALT were measured using an automated analyzer (CX5, Beckman Instruments Inc., Fullerton, CA).
[0224] Renal Morphology
[0225] Light Microscopy: Renal cortex fragments were fixed in Dubosq-Brazi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com