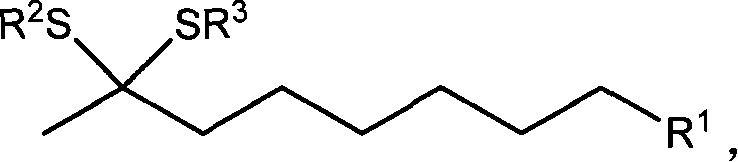
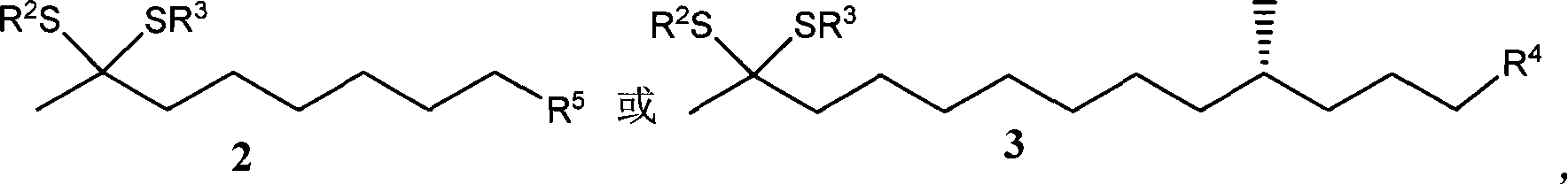
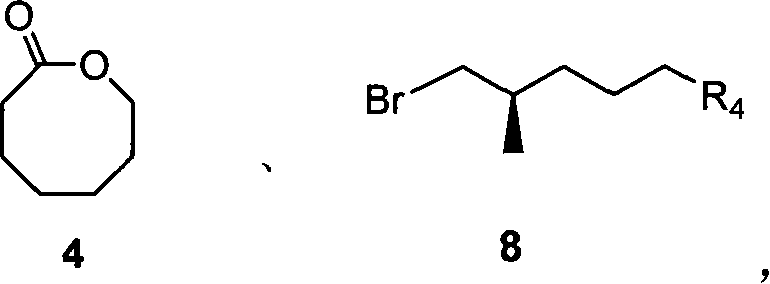
Compound of thioketal, synthetic method, and application in synthesizing pheromone of southern Diabrotica in optical purity
A synthesis method and compound technology, applied in the direction of organic chemistry and the like, can solve problems such as poor attraction of southern corn rootworm, and achieve the effects of fewer reaction steps, high yield and reduced pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of Example 1 Compound 2a
[0032]
[0033] A three-necked flask equipped with a constant pressure dropping funnel was treated with anhydrous and anaerobic treatment, added lactone 4 (342.5 mg, 2.67 mmol), 20 mL of absolute anhydrous ether, cooled to -78 ° C, and added dropwise 1.7 mL of MeLi ( 1.6M in Et 2 (0), stirred for 30 minutes after the completion of the dropwise addition, rose to room temperature, and TLC detected that the raw material disappeared, and the reaction system was poured into cold saturated NH 4 Quenched in Cl solution, separated the organic phase, back-extracted the aqueous phase with ether (2×15mL), combined the organic phases, washed with saturated brine, and anhydrous Na 2 SO 4 Dry and spin dry under reduced pressure, and the crude product is directly used in the next reaction.
[0034] The crude product obtained above was dissolved in 10 mL of dry CH 2 Cl 2 , add propanedithiol (0.4mL, 433mg, 4mmol), then ...
Embodiment 2
[0037] Synthesis of Example 2 Compound 2b
[0038]
[0039] A three-necked bottle equipped with a constant pressure dropping funnel, treated with anhydrous and anaerobic treatment. Compound 2a (1.17 g, 4.95 mmol) was dissolved in 35 mL of dry CH 2 Cl 2 , then add Et 3 N (1.1mL, 759mg, 7.5mmol), MsCl (0.6mL, 859mg, 7.5mmol) was added dropwise under ice-cooling. After 3 hours, TLC detected that the reaction was complete. Add 10 mL of water to quench the reaction, separate the organic phase, and the aqueous phase with CH 2 Cl 2 Back extraction (1×15mL), combined organic phases, washed with saturated brine, anhydrous Na 2 SO 4 Drying, spin-drying under reduced pressure, and flash column chromatography gave 1.48 g of a colorless oily substance 2b with a yield of 95%.
[0040] Compound 2b: C 12 h 24 o 3 S 3 ;FW312;
[0041] 1 H NMR (300MHz, CDCl 3 )δ: 4.22(t, J=6.9Hz, 2H), 3.00(s, 3H), 2.86-2.82(m, 4H), 1.98-1.86(m, 4H), 1.78-1.70(m, 2H), 1.61 ...
Embodiment 3
[0042] Synthesis of Example 3 Compound 2c
[0043]
[0044] A three-necked bottle equipped with a constant pressure dropping funnel, treated with anhydrous and anaerobic treatment. Compound 2a (1.17 g, 4.95 mmol) was dissolved in 35 mL of dry CH 2 Cl 2 , then add Et 3 N (1.1mL, 759mg, 7.5mmol), TsCl (1.43g, 7.5mmol) was added dropwise under ice-cooling. After 3 hours, TLC detected that the reaction was complete. Add 10 mL of water to quench the reaction, separate the organic phase, and the aqueous phase with CH 2 Cl 2 Back extraction (1×15mL), combined organic phases, washed with saturated brine, anhydrous Na 2 SO 4 Drying, spin-drying under reduced pressure, and flash column chromatography gave 1.85 g of a colorless oily substance 2c with a yield of 96%.
[0045] Compound 2c: C 18 h 28 o 3 S 3 ;FW 388;
[0046] 1 H NMR (300MHz, CDCl 3 )δ: 8.01(d, J=7.2Hz, 2H), 7.72(d, J=6.5Hz, 2H), 4.12(d, J=6.6Hz, 2H), 2.83-2.78(m, 4H), 2.43( s, 3H), 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com