Humanized single chain antibody for anti-human cytomegalovirus envelope glycoprotein
A human cytomegalovirus, envelope glycoprotein technology, applied in the direction of antiviral immunoglobulin, antibodies, metabolic diseases, etc., can solve the problems of high technical difficulty and high cost requirements, and achieve good specificity and good binding ability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0046] Example 1, clinical patient peripheral blood lymphocyte separation and RNA extraction
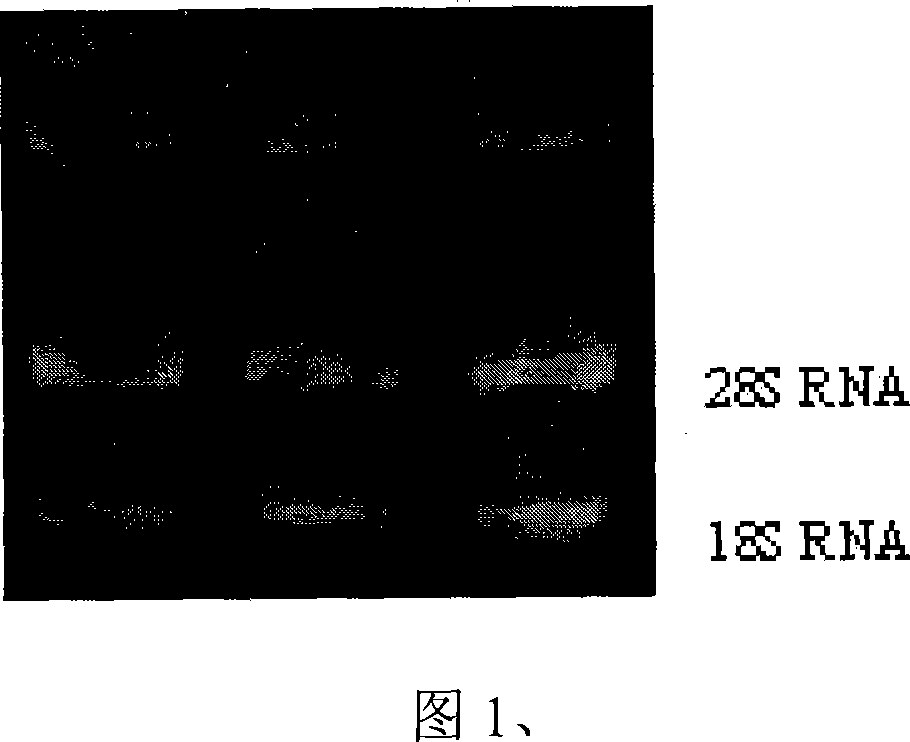
[0047] Take a 15ml centrifuge tube; add 8ml of lymphocyte separation solution to each tube. Gently superimpose 4ml of blood from each specimen on the separation medium; centrifuge at 3000rpm for 20 minutes; gently absorb the lymphocyte layer at the layered interface and centrifuge at 3000RPM for 20 minutes; gently suck off the supernatant, add 0.5ml Tripure and mix well. Extract RNA. The results of RNA extraction are shown in Figure 1
example 2
[0048] Example 2, Amplification of Humanized Single Chain Antibody Gene Fragment
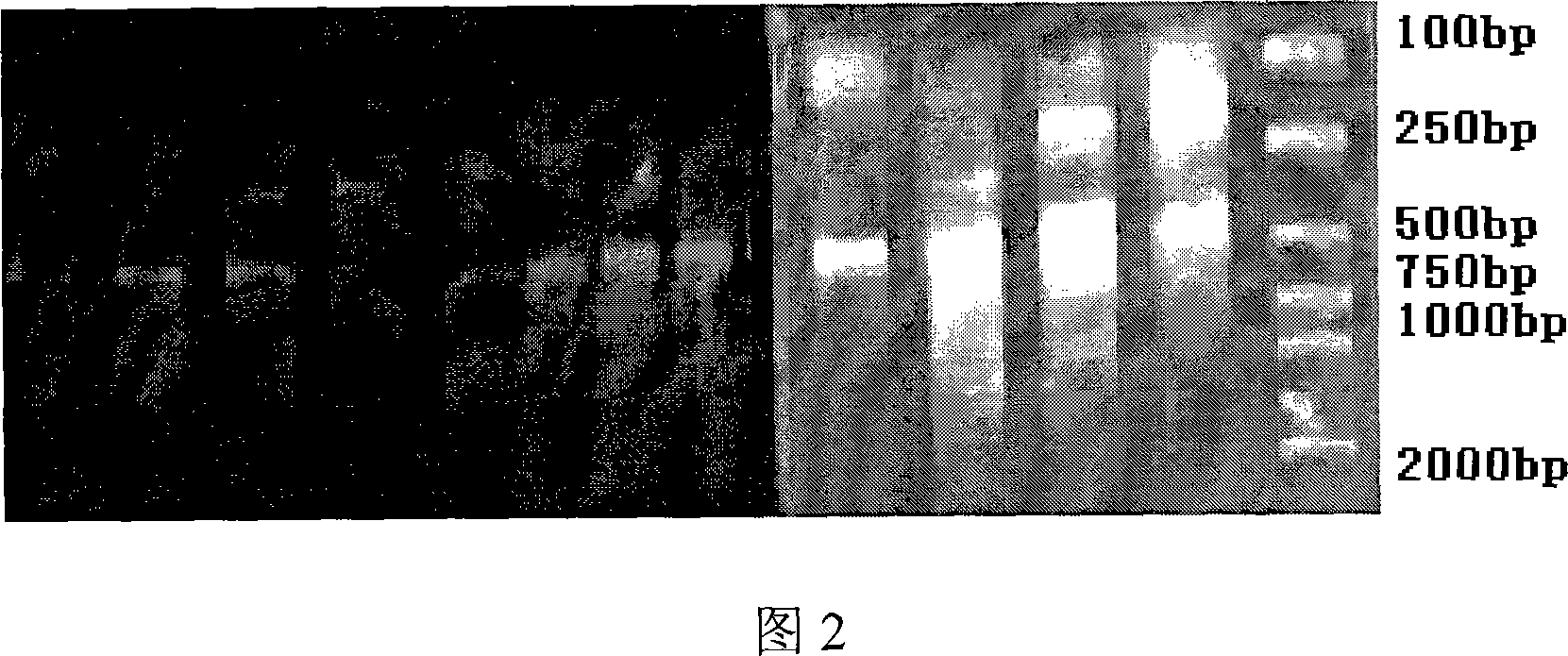
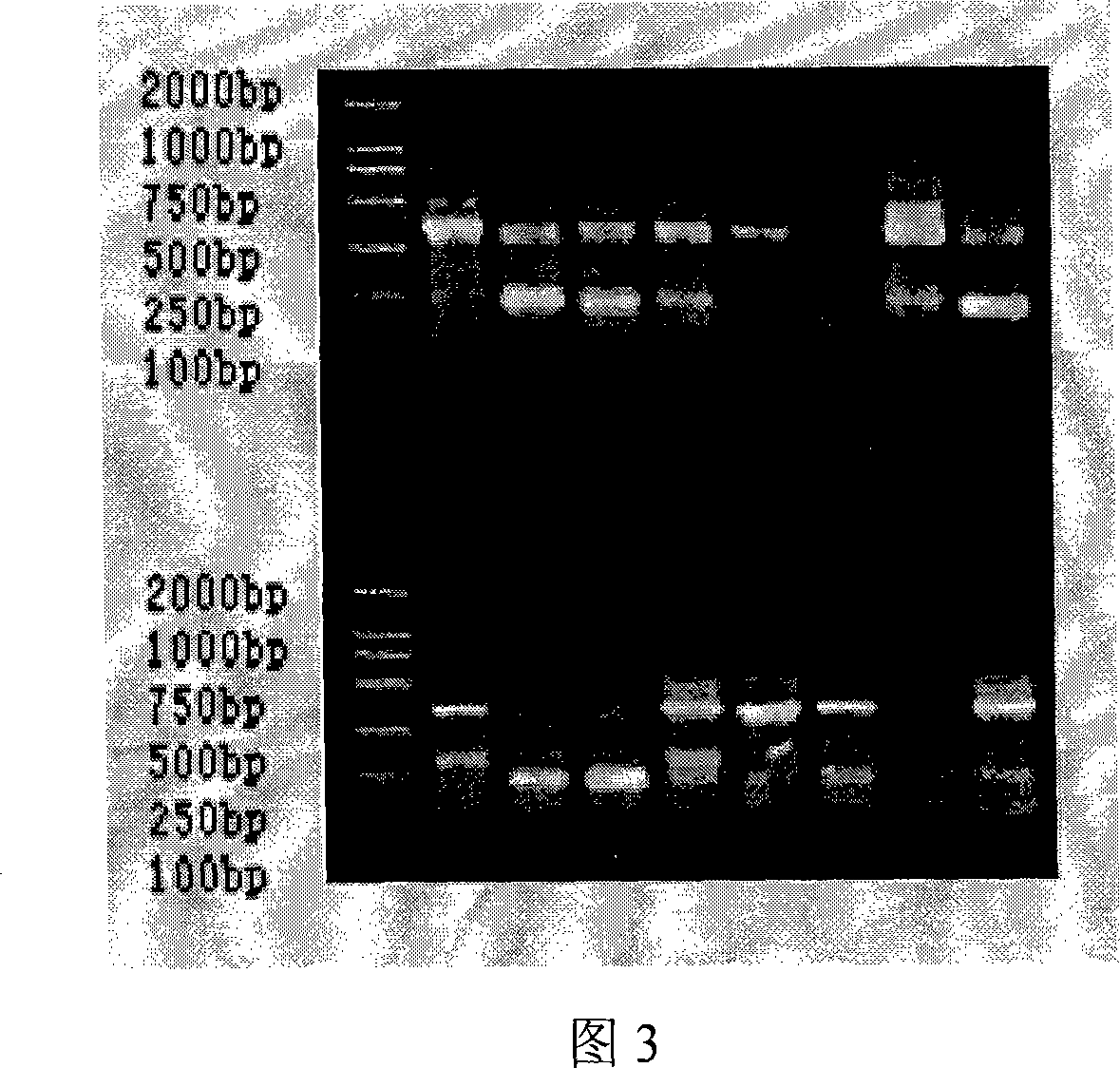
[0049] The RNA of the collected sample was extracted in Example 1, and the gene coding fragments of the antibody heavy chain and light chain variable regions were amplified by reverse transcription-polymerase chain reaction (RT-PCR), and the heavy chain and light chain variable regions were Assemble together by overlap-PCR. Among them, the reverse transcription reaction conditions: 30°C for 10 minutes; 42°C for 60 minutes; 99°C for 5 minutes; 4°C for 5 minutes. PCR conditions: 94°C for 5 min; 94°C for 30 sec, 56°C for 30 sec, 72°C for 30 sec, 35 cycles; 72°C for 10 min; 4°C for 10 min. Overlap-PCR conditions: 94°C for 5 min; 94°C for 30 sec, 56°C for 30 sec, 72°C for 2 min, cycle 25 times; 72°C for 10 min; 4°C for 10 min. The obtained results are shown in Figures 2-5.
example 3
[0050] Example 3, Construction of Antibody Library
[0051] The recovered and purified overlap-PCR product and the carrier plasmid pComb3X were respectively digested with SfiI, and the digested single-chain antibody fragment and vector were recovered and purified, ligated with DNA ligase, and incubated at room temperature for 4 hours to overnight. The connected samples and the corresponding number of electroporation cups were placed in an ice bath for 10 min. At the same time, take electrocompetent bacteria and dissolve them on ice. Mix the connected samples with competent bacteria and add them to the electroporation cup. Ice bath for 1 minute, the electroporator was set to 25μF, 2.5kV, 200Ω, and the duration was about 4-5 milliseconds. Rinse the electro-cup immediately with 1, 2, and 2ml of SOC medium in batches, mix the washing solution in a 50ml test tube, and shake at 37°C and 250rpm for 1 hour. Add 10ml of preheated SB medium, 3ul100mg / ml carbenzyl (if using XL1-blue, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com