Process for producing benzyl chlorides chemical compound
A compound and material technology applied in the production of benzyl chloride compounds, which can solve the problems of product coking, high temperature heat source, high energy consumption, etc., and achieve the effects of reaction operation control, high economic benefits, and easy reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
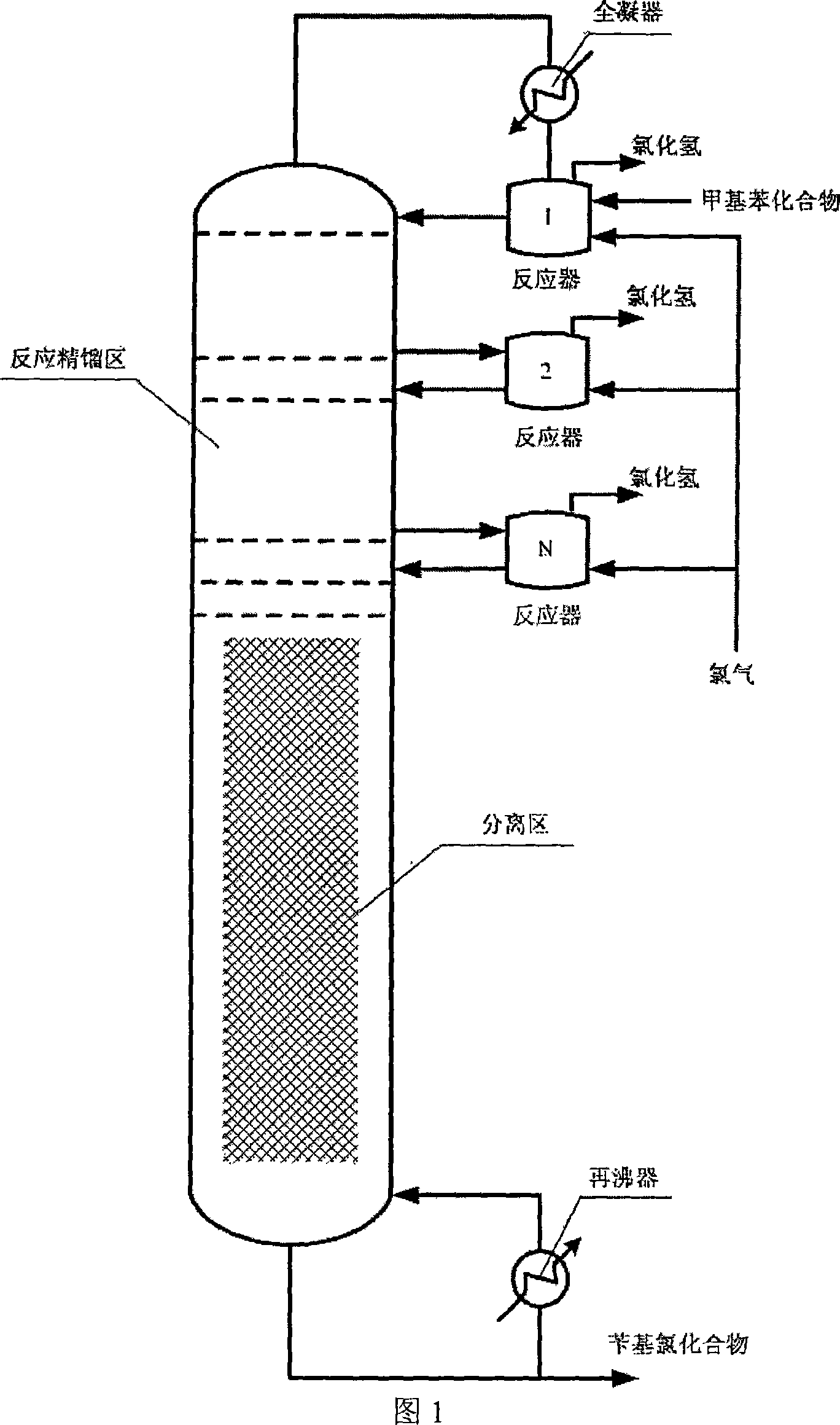
[0020] Toluene and chlorine feed are both 1.40kmol / h, the distribution ratio of chlorine in the three reactors is: 0.45:0.32:0.23, the amount of condensation at the top of the tower: 1000L / h, the temperature of the chlorination reactor is 105°C, rectification The operating pressure of the tower is 0.025MPa, the number of trays in the rectification zone is 9, and the number of trays in the separation zone is 30. After reactive distillation, the molar composition of the material in the tower kettle is: 0.48% toluene, 99.25% benzyl chloride, 0.26% benzylidene dichloride, and 0.04% cyclochlorotoluene. The selectivity of benzyl chloride is 98.96%.
Embodiment 2
[0022] Both toluene and chlorine feed are 1.40 kmol / h, the distribution ratio of chlorine in the three reactors is: 0.45:0.32:0.23, and the condensation at the top of the tower is 1300 L / h. , the temperature of the chlorination reactor is 80°C, the operating pressure of the rectification tower is 0.01MPa, the number of trays in the rectification zone is 6, and the number of trays in the separation zone is 26. After reactive distillation, the molar composition of the material in the tower kettle is: 0.46% toluene, 99.29% benzyl chloride, 0.21% benzylidene dichloride, and 0.04% cyclochlorotoluene. The selectivity of benzyl chloride is 99.08%.
Embodiment 3
[0024]Both p-chlorotoluene and chlorine feed are 1.40 kmol / h, the distribution ratio of chlorine in the four reactors is: 0.38:0.28:0.20:0.18, the amount of condensation at the top of the tower: 1000L / h, and the temperature of the chlorination reactor is 105 °C, the operating pressure of the rectification tower is 0.004MPa, the number of trays in the rectification zone is 8, and the number of trays in the separation zone is 30. After reactive distillation, the molar composition of the material in the tower kettle is: p-chlorotoluene 0.43%, p-chlorobenzyl chloride 99.07%, p-chlorobenzylidene dichloride 0.48%, cyclodichlorotoluene 0.02%. The selectivity to chlorobenzyl chloride is 98.59%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com