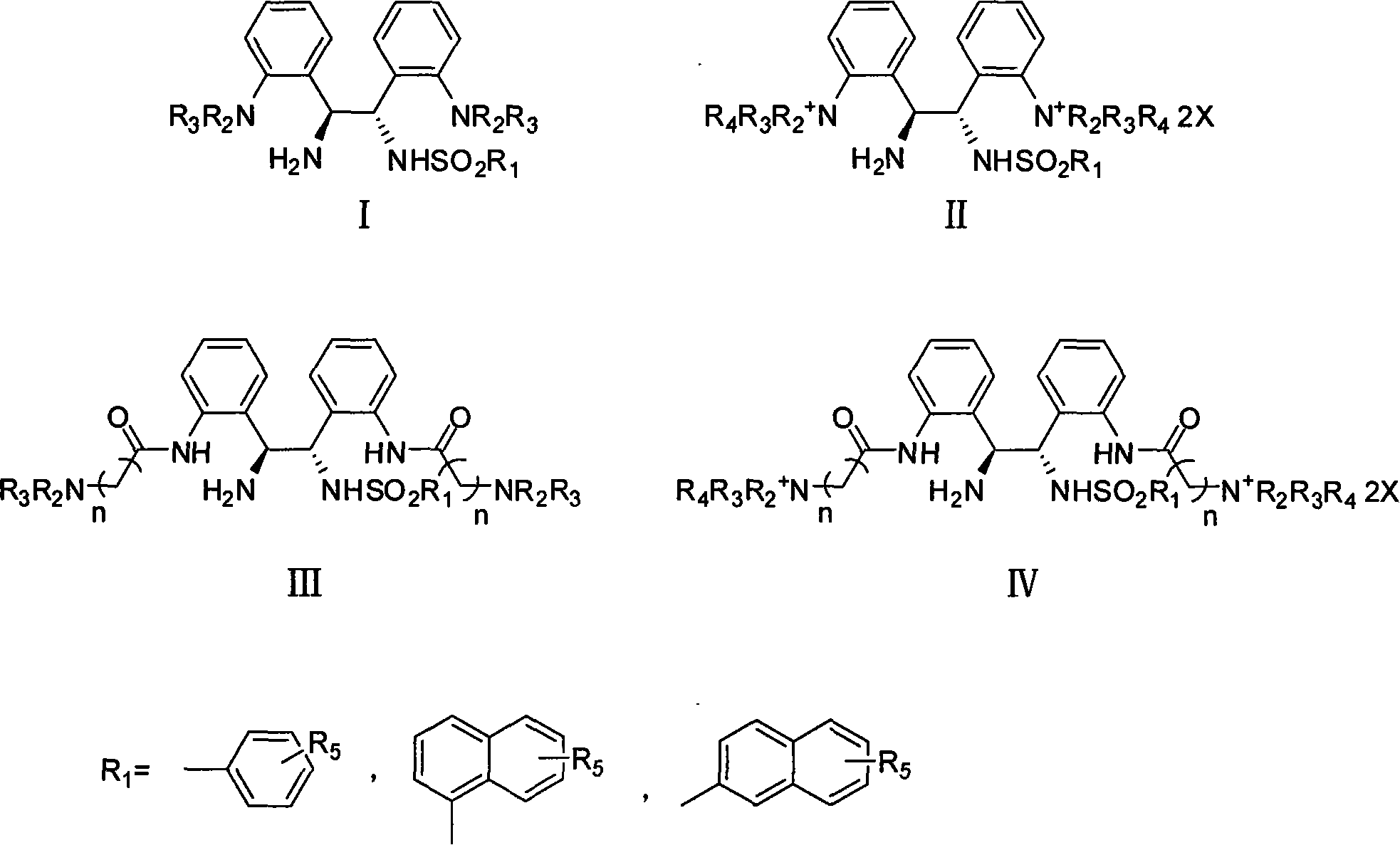
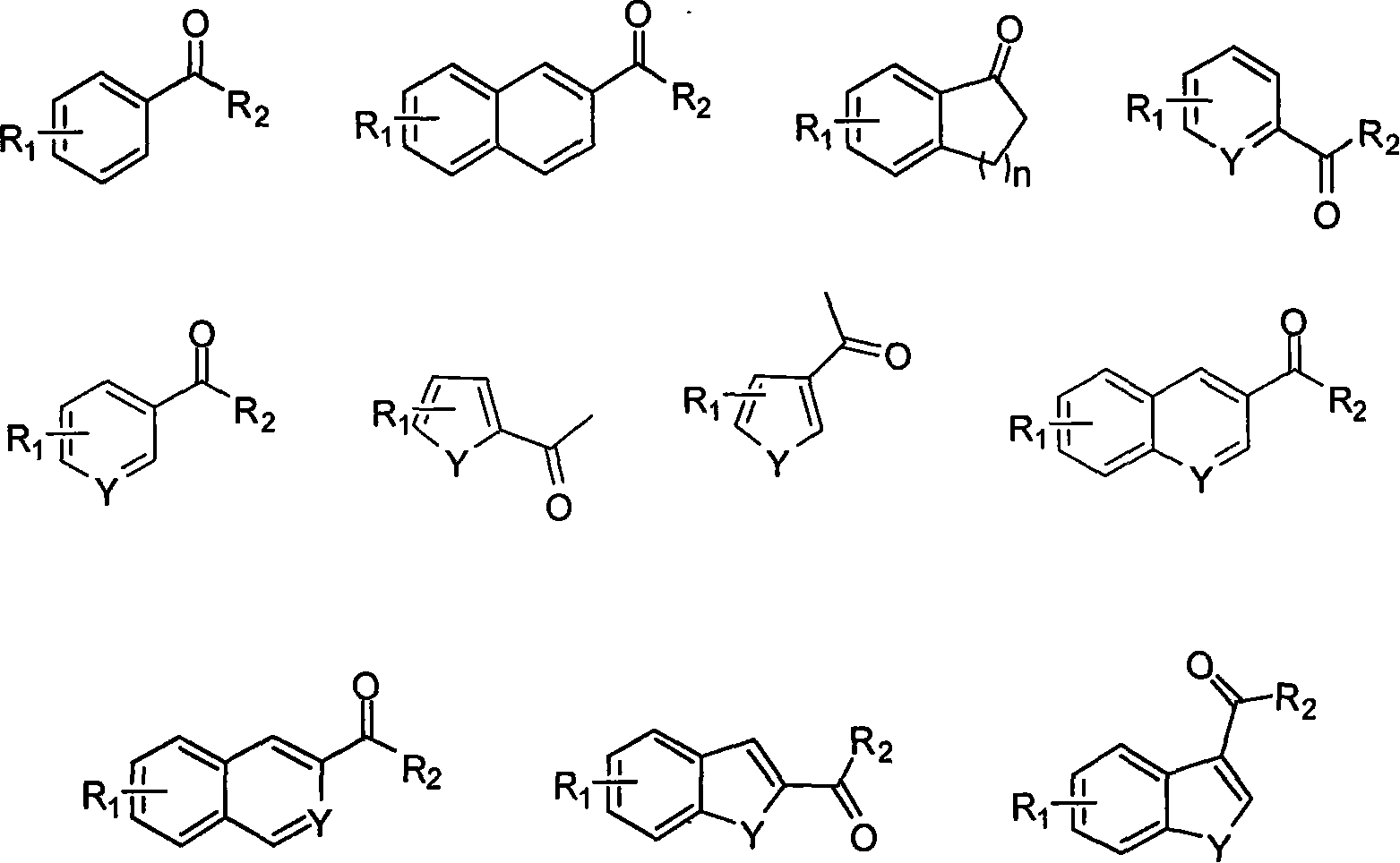
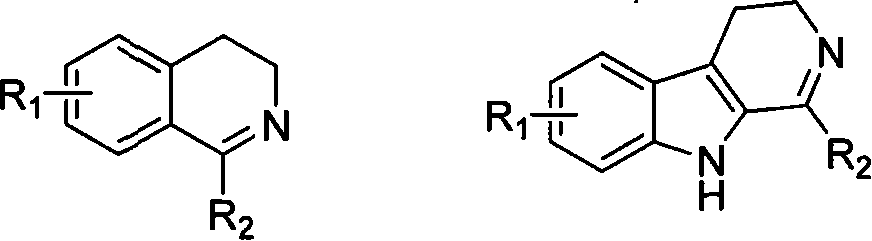Soluble chiral diamino derivative, its production and use
A chiral diamine and water-soluble technology, which is applied in the field of chiral diamine and its derivatives, can solve the problems of inability to form micelles or capsules, unfavorable special microreaction environment and stable holes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Asymmetric transfer hydrogenation of acetophenone in aqueous phase catalyzed by complexes of ruthenium and I
[0074] 1.2mg (0.002mmol) of [RuCl 2 (p-cymene)] 2 and 1.7mg (0.0044mmol) of water-soluble ligand N-tosylated-1,2-bis-(2'-aminophenyl)-1,2-ethylenediamine were added to 1mL of water and activated at 40°C 1 hour. At room temperature, 208mg (2.0mmol) HCOONa·2H 2 O and 48 μL (0.4 mmol) of acetophenone were added to the system and reacted for 0.5 hours. Then use CH 2 Cl 2 After extraction several times, the extract was dried with anhydrous sodium sulfate, filtered and concentrated, and the conv. and ee values were determined by GC (33% conv., 95% ee).
Embodiment 2
[0075] The asymmetric transfer hydrogenation reaction of acetophenone in the water phase that the complex of iridium and I catalyzes the embodiment two
[0076] 1.6mg (0.002mmol) of [IrCl 2 (cp * )] 2 and 1.7mg (0.0044mmol) of water-soluble ligand N-tosylated-1,2-bis-(2'-aminophenyl)-1,2-ethylenediamine were added to 1mL of water and activated at 40°C 1 hour. At room temperature, 208mg (2.0mmol) HCOONa·2H 2 O and 48 μL (0.4 mmol) of acetophenone were added to the system and reacted for 0.5 hours. Then use CH 2 Cl 2 After extraction several times, the extract was dried with anhydrous sodium sulfate, filtered and concentrated, and the conv. and ee values were determined by GC (29% conv., 95% ee).
[0077] Asymmetric transfer hydrogenation of acetophenone in aqueous phase catalyzed by complexes of rhodium and I
Embodiment 3
[0079] 1.3mg (0.002mmol) of [RhCl 2 (cp * )] 2 and 1.7mg (0.0044mmol) of water-soluble ligand N-tosylated-1,2-bis-(2'-aminophenyl)-1,2-ethylenediamine were added to 1mL of water and activated at 40°C 1 hour. At room temperature, 41.6mg (0.4mmol) HCOONa·2H 2 O and 48 μL (0.4 mmol) of acetophenone were added to the system and reacted for 0.5 hours. Then use CH 2 Cl 2 After extraction several times, the extract was dried with anhydrous sodium sulfate, filtered and concentrated, and the conv. and ee values were determined by GC (86% conv., 97% ee).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com