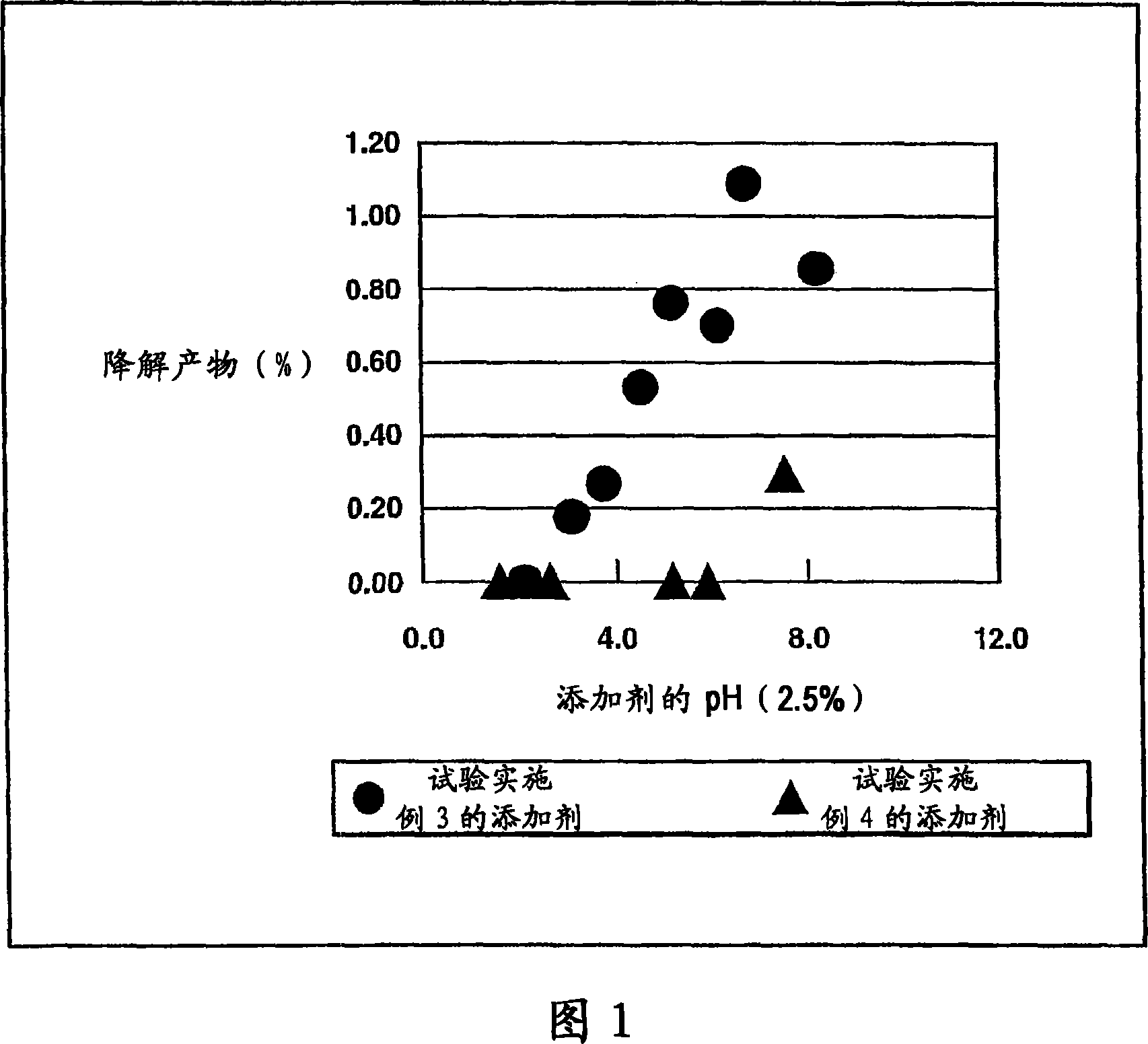
Matrix type sustained-release preparation
A technology for drugs and dementia, applied in pharmaceutical formulations, drug delivery, active ingredients of heterocyclic compounds, etc., to achieve the effect of low burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Add an appropriate amount of purified water and mix with 300 mg of donepezil hydrochloride (Eisai Co. Ltd.), 375 mg of ethyl cellulose (Ethocel 10FP, Dow Chemical Company), 1500 mg of Eudragit L100-55 (Röhm GmbH & Co. KG) and 795 mg of lactose were mixed, and the mixture was heated and dried in a constant temperature room. 30 mg of magnesium stearate (Mallinckrodt Baker, Inc.) was added and mixed with the dry granules. 200 mg of this mixture was taken and tableted with Autograph AG5000A (Shimazu Corporation) to obtain tablets with a diameter of 8 mm containing 20 mg of donepezil hydrochloride.
Embodiment 2
[0084] Add an appropriate amount of purified water and mix with 20 mg of donepezil hydrochloride (Eisai Co. Ltd.), 500 mg of Eudragit L100-55 (Rhm GmbH & Co. KG), 1000 mg of lactose and 500 mg of ethylcellulose (Ethocel 10FP, Dow Chemical Company) were mixed, and the mixture was heated and dried in a constant temperature dryer to obtain granules containing donepezil hydrochloride in an amount of about 1% of the total weight of the granules.
Embodiment 3
[0086]20 mg of citric acid was dissolved in an appropriate amount of purified water and mixed with 20 mg of donepezil hydrochloride (Eisai Co. Ltd.), 500 mg of Eudragit L100-55 (Rhm GmbH & Co. KG), 1000 mg of lactose and 500 mg of Ethyl cellulose (Ethocel 10FP, Dow Chemical Company) was mixed, and the mixture was heated and dried in a constant temperature dryer to obtain granules containing about 1% of donepezil hydrochloride based on the total weight of the granules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com