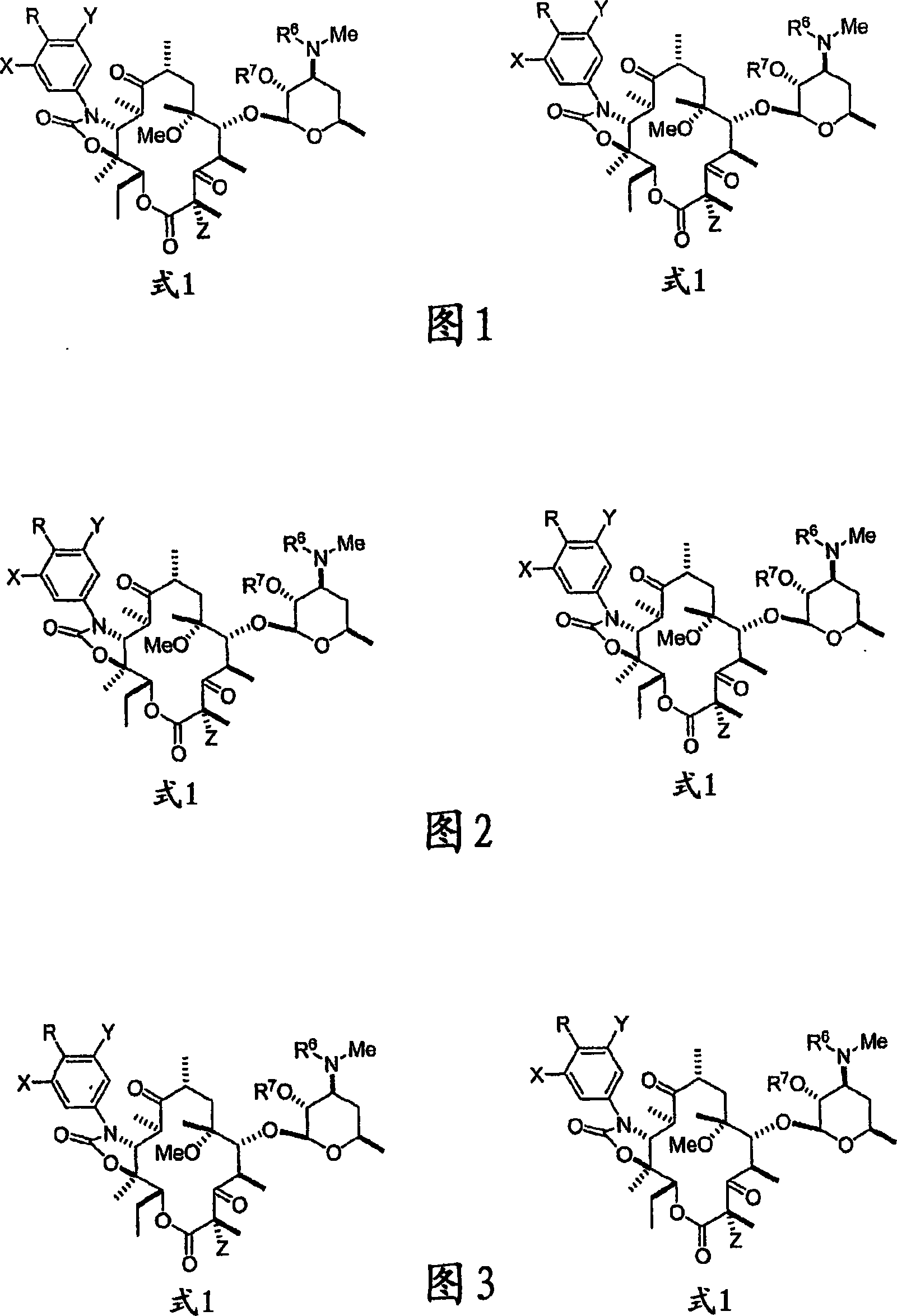
Erythromycin derivatives as antibacterial agents
A technology of solvates and halogens, applied in the field of new antibiotics, can solve the problems of not having a direct bonded benzene ring, not disclosing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0144] 4″-O-(allyloxycarbonyl)-N-demethyl clarithromycin 11,12-carbonate-2′,3′-aminomethyl Ethyl acetate (5)
[0145] Clarithromycin (4, 5.00 g, 6.69 mmol) was dissolved in dichloromethane (100 mL), and pyridine (6.30 mL, 77.9 mmol) and phosgene (20.0 mL, 38.0 mmol in 20% toluene) were added. The reaction mixture was stirred at room temperature for 5 hours. Allyl alcohol (9.00ml, 132mmol) was added and stirring continued for an additional 30 minutes (yellow solution). Aqueous sodium hydroxide solution was added, and the product was extracted with dichloromethane. The combined organic layers were washed with water and brine, dried over magnesium sulfate and filtered. Evaporation of the filtrate and chasing toluene gave a pale yellow solid which was recrystallized from toluene; 5.07 g (87%) of allyl carbonate 5 was obtained as a white solid; mp.: 307-310° C. (toluene). (C 43 h 67 NO 17 Calculated: C, 59.36; H, 7.76. Found: C, 60.01; H, 7.36%). HRMS, ESIpos.: M+Na + =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com