Preparation for anti human B7-H3 monoclonal antibody and application thereof
A monoclonal antibody, B7-H3 technology, applied in the direction of anti-animal/human immunoglobulin, antibody, measuring device, etc., can solve the difficulties of less cell surface, limited in-depth study of DC function, DC separation and purification and phenotypic identification And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of anti-human B7-H3 monoclonal antibody
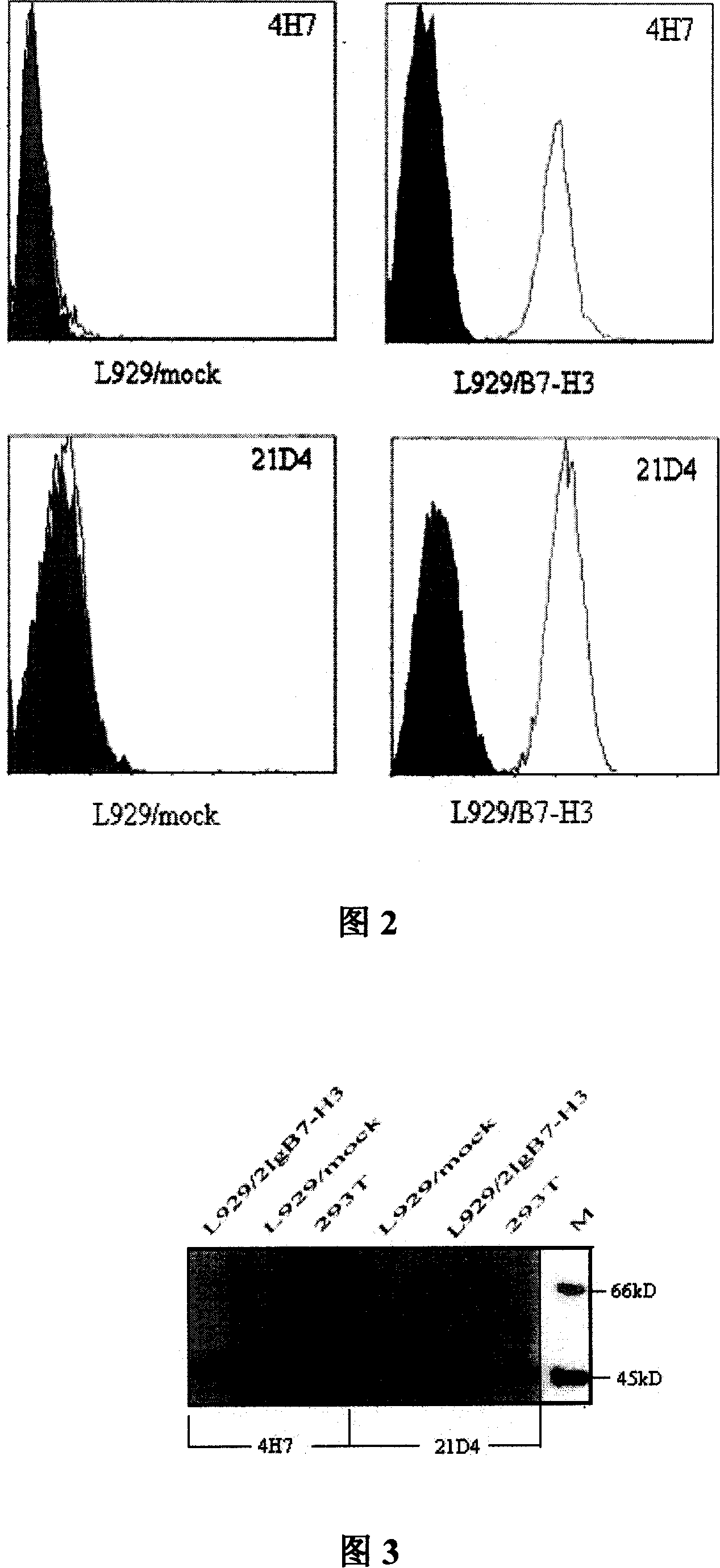
[0043] This example describes the preparation of the anti-human B7-H3 monoclonal antibodies 4H7, and 21d4 of the present invention.
[0044] (1) Establishment of transgenic cells L929 / B7-H3 and L929 / mock
[0045] (a) Cloning of human B7-H3 gene: TRIZOL reagent (Gibco, BRL) was used to extract the total RNA of antigen-induced mature DC. According to the method recommended in the instruction manual of the MBI reverse transcription kit, the mRNA was reverse transcribed into cDNA. Take 5 μl of cDNA as a template, use the upstream primer 5'-AA CTG CAGATG GAA AGG GTC CAA CCC-3', and the downstream primer 5'-CG GGA TTC TCA AAGGAC ACA GAA TTC-3' for PCR amplification (denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min, and extension at 72°C for 5 min after 30 cycles). After purification, at T 4 The PCR product was directly connected to the pMD18-T vector under the action of ...
Embodiment 2
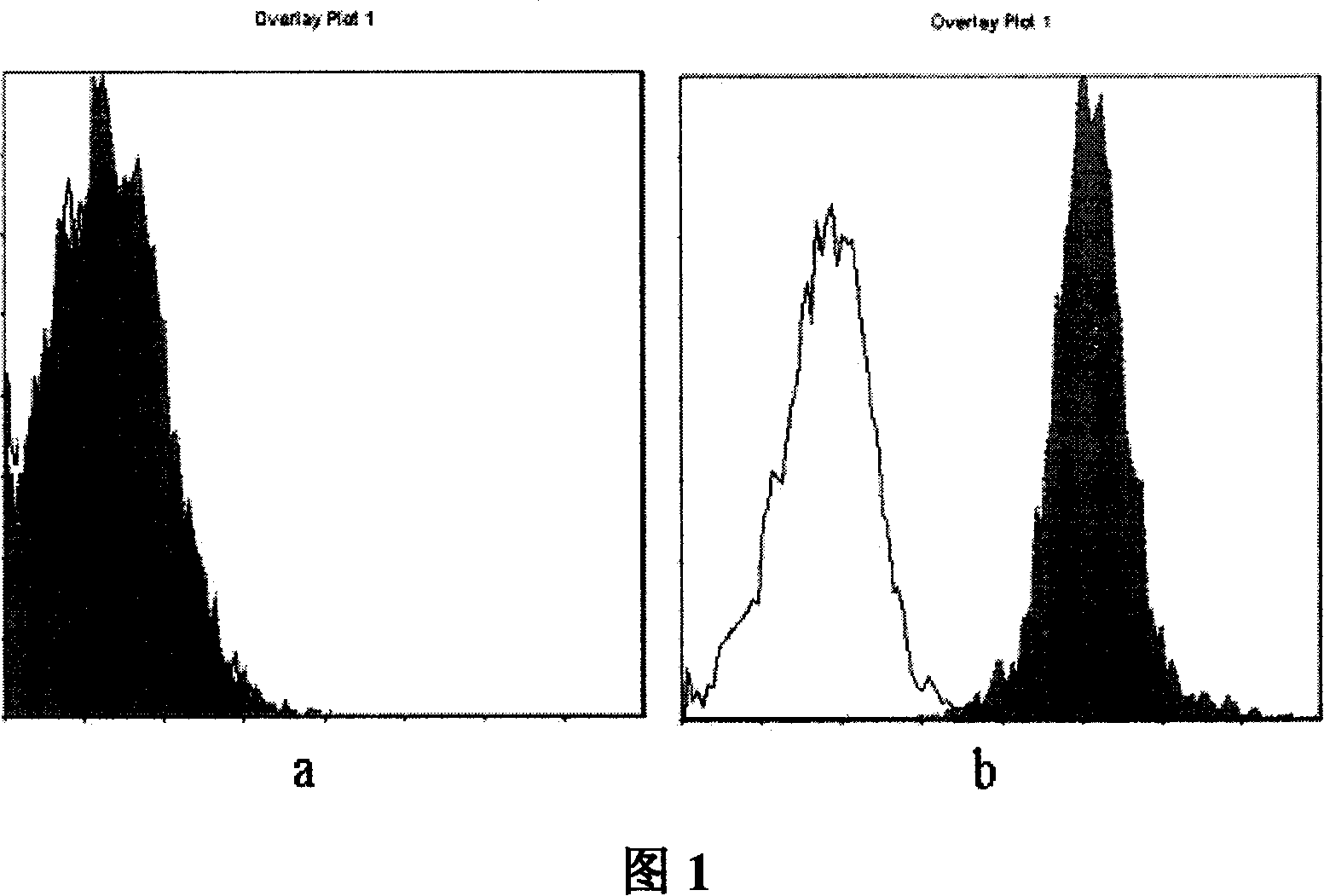
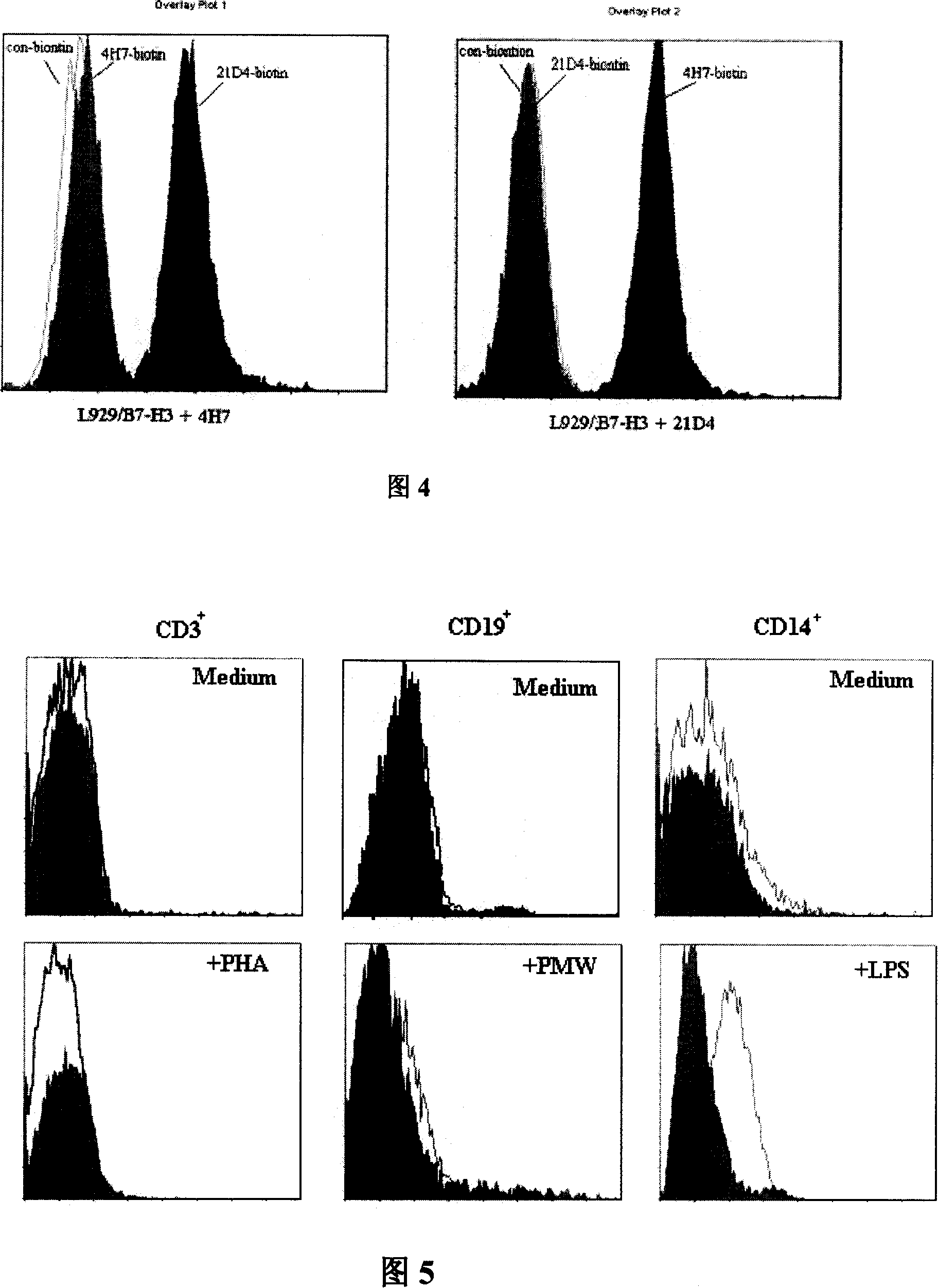
[0056] Example 2: Recognition ability of monoclonal antibody 21D4 to B7-H3 molecules expressed on the surface of Mo-DCs
[0057] 1) Mononuclear cells (PBMC) were routinely isolated from peripheral blood of healthy people. After washing twice with RPMI-1640, the cell density was adjusted to 3×10 with RPMI-1640 containing 10% FCS. 6 / ml, added to 6-well culture plate (2ml / well). then 5% CO 2 , 37°C for 2 hours. After aspirating the suspended cells, add GM-CSF (100ng / ml), IL-4 (50ng / ml), 10% FCS, 0.02mmol / LL-glutamine, 5×10 -5 / mol / L2-ME RPMI-1640 continued to be cultured, and the medium was changed every 3 days. After 7 days of culture, 5C11 (5 μg / ml) or TNF-α (10 ng / ml) was added for induction for 3 days.
[0058] 2) DCs in different developmental stages of each induction group were collected, and the expression of B7-H3 on the cell surface was analyzed by flow cytometry. The results showed that: B7-H3 showed extremely stable high-level expression on Mo-DCs. The expressio...
Embodiment 3
[0059] Example 3: Relationship between B7-H3 expression on tumor cells and tumor prognosis
[0060] 1) Case source
[0061]102 specimens of gastric cancer were obtained from gastric cancer surgery patients admitted in the Third Affiliated Hospital of Soochow University during 1998-1999. Among them, there were 75 males and 27 females. Aged 28-77 years old, with an average of 55 years old. Tumors were located in the mucosa and submucosa in 10 cases, located in the superficial muscular layer in 20 cases, and the tumor infiltration depth reached or exceeded the deep muscular layer in 72 cases. Histological types are divided into: differentiated adenocarcinoma (papillary adenocarcinoma, well-differentiated tubular adenocarcinoma, moderately differentiated tubular adenocarcinoma) and poorly differentiated adenocarcinoma (poorly differentiated adenocarcinoma, signet ring cell carcinoma, mucinous cell carcinoma). All patients did not receive chemotherapy or radiotherapy before oper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com