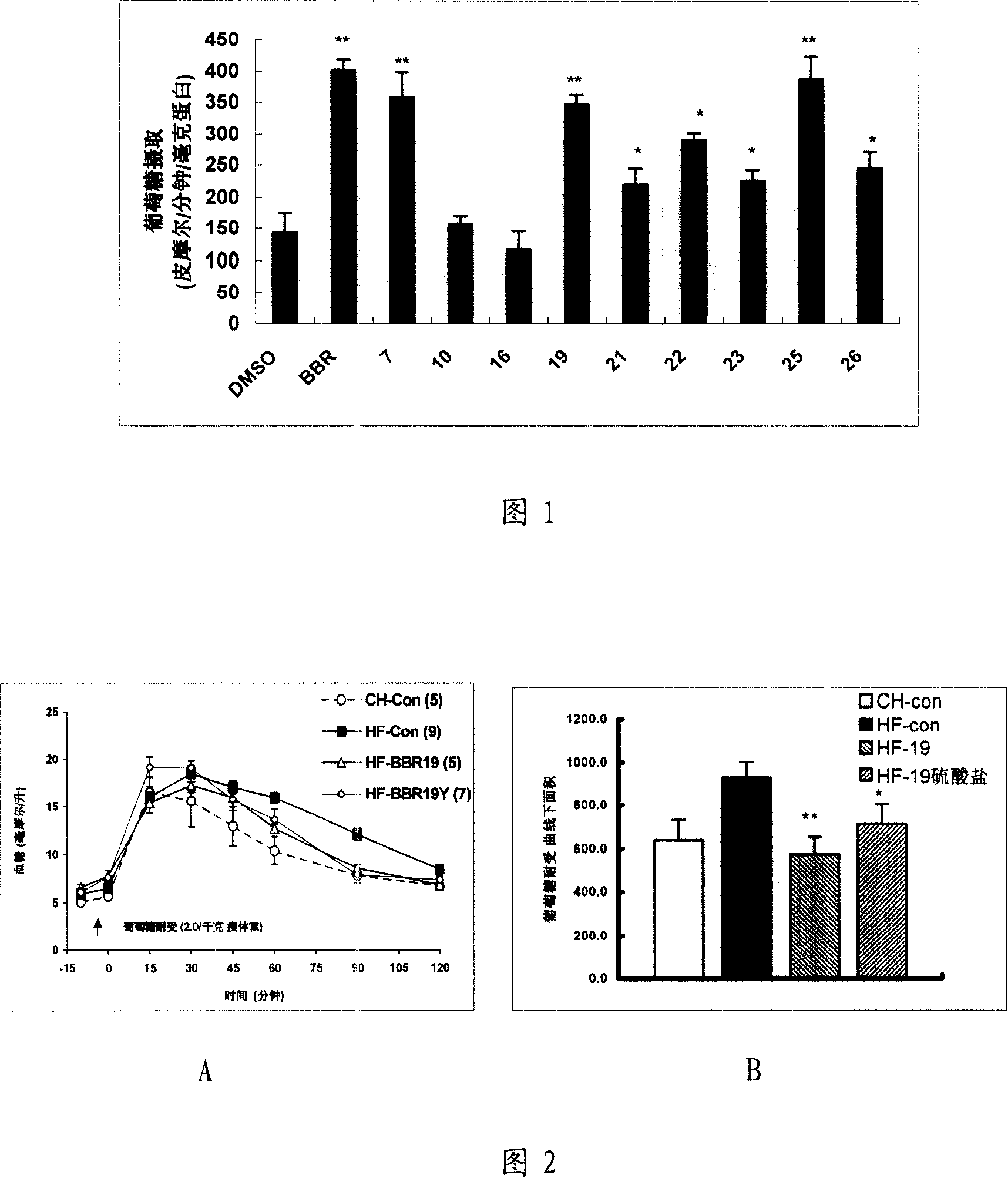
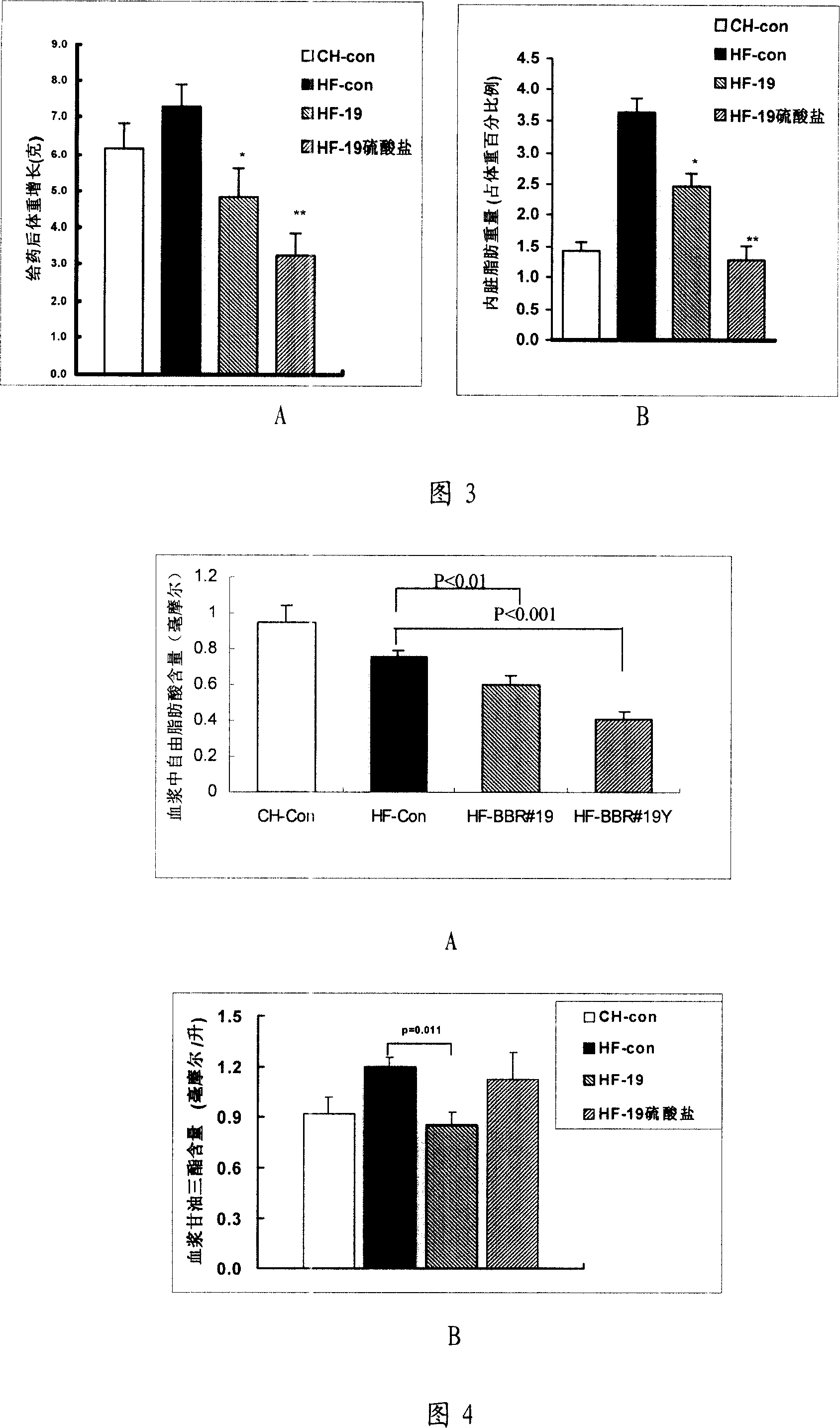
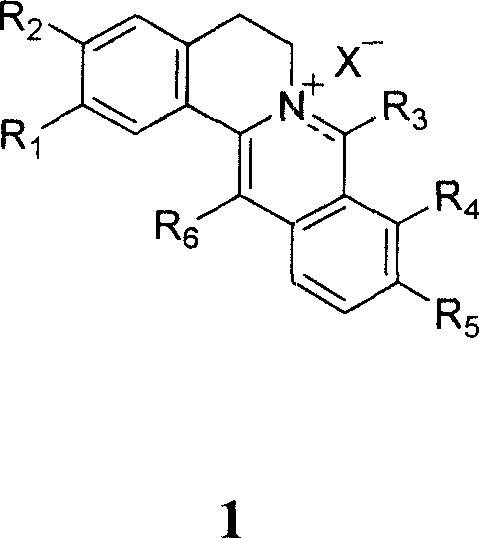
13, 13a- dihydro berberine derivant, pharmaceutical composition and uses of the same
A technology of dihydroberberine and its derivatives, applied in 13 fields, can solve problems such as poor solubility of compounds, difficulty in reaching the body through oral absorption, and restrictions on further application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] Preparation of compound 2
[0063] 8-Acetonyl dihydroberberine (3 g) and methyl iodide were dissolved in 100 mL of dichloromethane, heated to 100° C. under pressure and reacted for 3 hours. After the reaction, the solid by-product was filtered off, and the filtrate was evaporated to dryness under reduced pressure. The residue was recrystallized in methanol to obtain compound 2 (1.53 g, 48%).
[0064] Compound 2, C 21 h 20 INO 4 , MW: 477; yellow crystals, easily soluble in mixed solvents of chloroform and methanol.
[0065] 1 H NMR (300MHz, DMSO-d 6 ): δ9.89(1H, s, H-8), 8.20(1H, d, J=9.0Hz, H-12), 8.19(1H, d, J=9.0Hz, H-11), 7.48(1H , s, H-1), 7.15 (1H, s, H-4), 6.18 (2H, s, -OCH 2 O-), 4.80 (2H, m, H-6), 4.10 (3H, s, -OCH 3 ), 4.09 (3H, s, -OCH 3 ), 3.15 (2H, m, H-5), 2.92 (3H, s, -CH 3 ).
[0066] Preparation of compound 3
[0067]8-Acetonyl dihydroberberine (1.5g) and ethyl bromide were dissolved in 100mL of dichloromethane, heated to 100°C under pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com