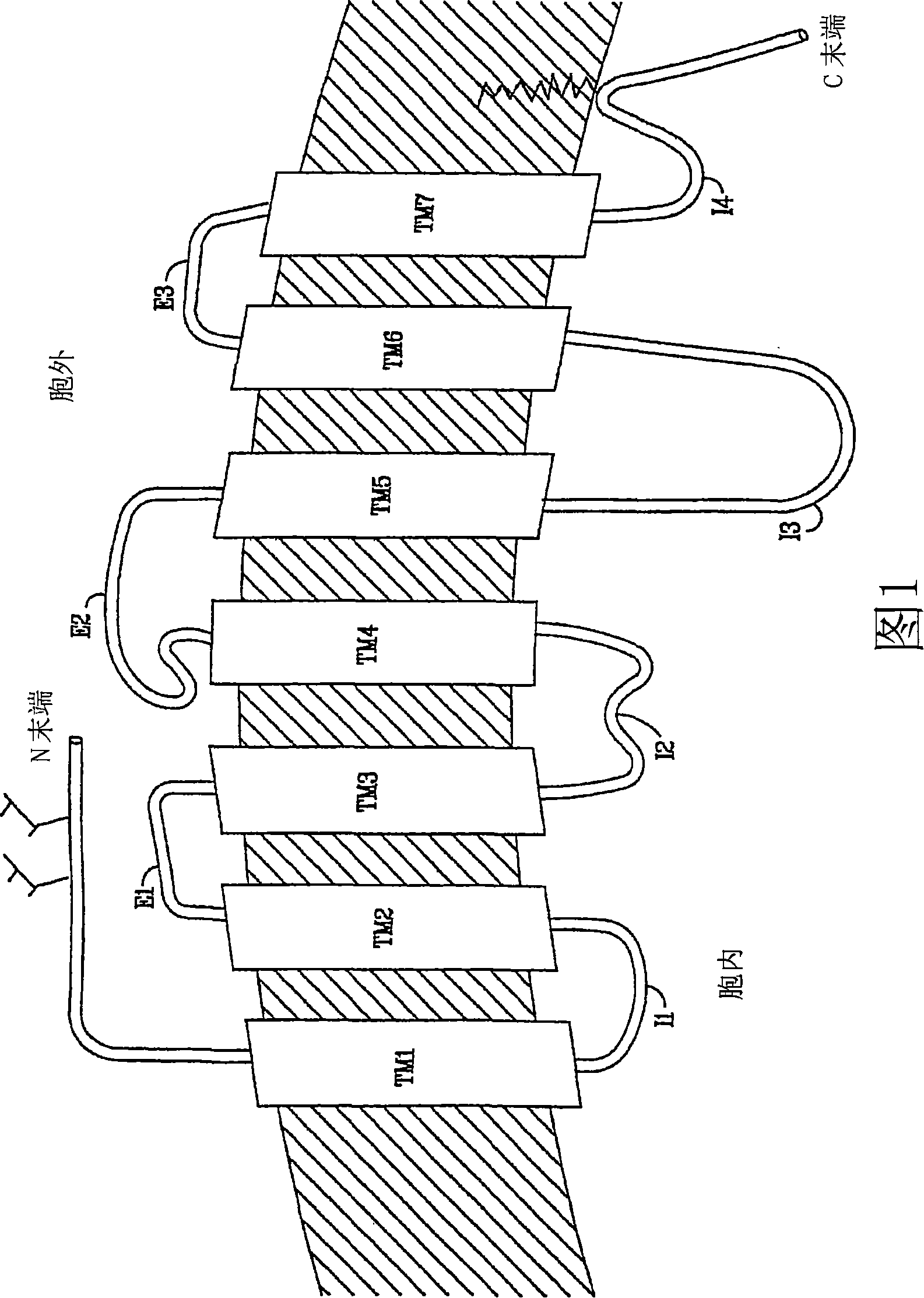
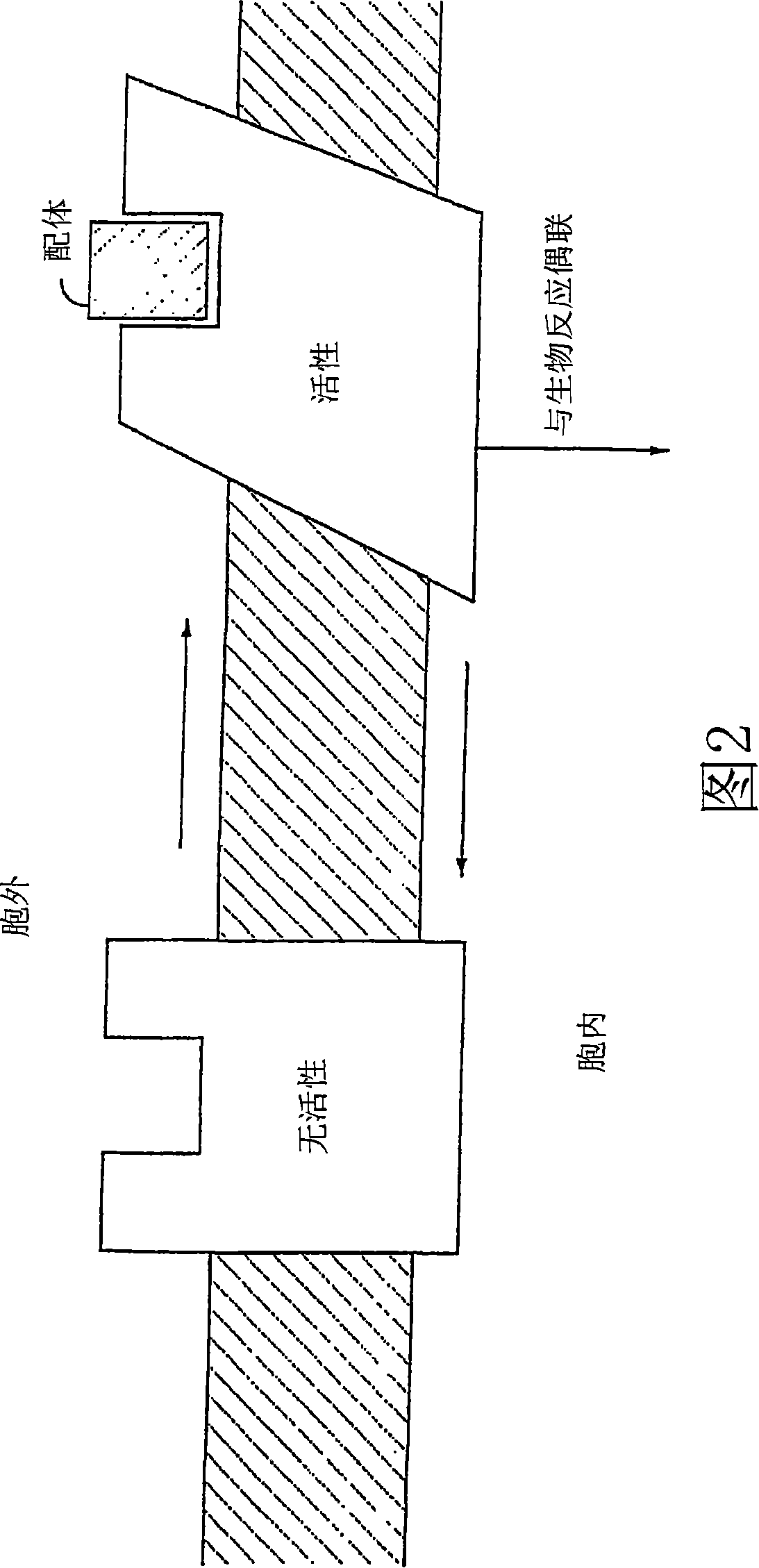
Diaryl and arylheteroaryl urea derivatives as modulators of the 5-ht2a serotonin receptor useful for the prophylaxis or treatment of progressive multifocal leukoencephalopathy
A leukoencephalopathy, alkyl technology, applied in the field of prevention or treatment of progressive multifocal leukoencephalopathy, can solve problems such as the absence of effective therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0586] The active content 125 I. The synthetic methods for incorporating the target molecule include:
[0587] A. Sandmeyer reaction and similar reactions-this procedure converts aryl or heteroaryl amines into diazonium salts, such as tetrafluoroborate and then Na 125 I convert it into 125 I labeled compound. The representative procedure was reported by Zhu, D.-G. and colleagues in J. Org, Chan. 2002, 67, 943-948.
[0588] B. Phenol ortho 125 Iodination reaction-this procedure allows incorporation at the ortho position of phenol 125 I, as reported by Collier, T.L. and colleagues in J. Labeled Compd Radiopharm 1999, 42, S264-S266. .
[0589] C. Take 125 I replace aryl and heteroaryl bromide-this method is usually a two-step method. The first step is, in the trialkyl tin halide or hexaalkyl ditin [for example (CH 3 ) 3 SnSn(CH 3 ) 3 ] In the presence of, for example, a reaction catalyzed by Pd [that is, Pd(Ph 3 P) 4 ] Or the aryl or heteroaryl bromide is converted to the correspondi...
example 1
[0596] Receptor cDNA
[0597] A. Constitutively activated 5-HT 2C Construction of receptor cDNA
[0598] 1. Endogenous human 5-HT 2C
[0599] Encoding endogenous human 5-HT 2C Receptor cDNA is made from human brain poly A + RNA was obtained by RT-PCR. The 5'and 3'primers are derived from the 5'and 3'untranslated regions and contain the following sequences:
[0600] 5'-GACCTCGAGGTTGCTTAAGACTGAAGCA-3' (SEQ.BD.NO.:1);
[0601] 5'-ATTTCTAGACATATGTAGCTTGTACCGT-3' (SEQ. ID. NO.: 2).
[0602] PCR is using TaqPlus TM Precision polymerase (Stratagene) or rTth TM Polymerase (Perkin Elmer) and the buffer system provided by the manufacturer, 0.25 μM each primer, and 0.2 mM four (4) nucleotides each were performed. The cycling conditions were 30 cycles, 1 minute at 94°C; 1 minute at 57°C and 2 minutes at 72°C. The 1.5 kb PCR fragment was digested with Xho I and XbaI and subcloned into the Sal I-Xba I site of pBluescript.
[0603] The cDNA thus obtained was fully sequenced and found to correspon...
example 2
[0647] Receptor expression
[0648] A.pCMV
[0649] For the purpose of producing related polypeptides in cells, a variety of expression vectors in this technology can be used. One suitable vector is pCMV used in certain embodiments. In accordance with the Budapest Treaty (Budapest Treaty) on the international recognition of the microbial preservation patent procedures, this vector was stored in the American Type Culture Collection (ATCC) (10801 University Blvd., October 13, 1998) Manassas, VA20110-2209USA). DNA is tested by ATCC and confirmed to be usable. ATCC awarded pCMV the following deposit number: ATCC#203351. See Figure 8 . Those skilled in the art will readily be apparent to other suitable expression vectors.
[0650] B. Transfection procedure
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com