Alpha ketoamide compounds as cysteine protease inhibitors
Technology of a compound, alkyl, in the field of alpha ketoamide compounds as cysteine protease inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
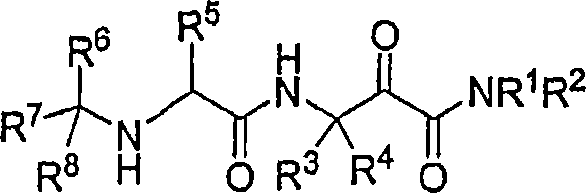
[0107] I. Certain compounds of formula (I) within the broadest ranges set forth in the Summary of the Invention are preferred. E.g:
[0108] (A) A preferred group of compounds is that wherein:
[0109] R 1 is hydrogen or methyl, preferably hydrogen;
[0110] R 2 is cyclopropyl, 1-phenethyl[-CH(C 6 h 5 )CH 3 ], or 1H-pyrazol-5-yl; preferably cyclopropyl.
[0111] (1) Among the above-mentioned preferred group (A) and the more preferred groups contained therein, the more preferred group of compounds is that wherein: R 3 is hydrogen and R 4 is an alkyl group, preferably methyl, ethyl, propyl or butyl, more preferably R 4 is ethyl or propyl.
[0112] (2) Among the above-mentioned preferred group (A) and the more preferred groups contained therein, the more preferred group of compounds is that wherein: R 3 is an alkyl group, preferably methyl or ethyl and R 4 is an alkyl group, preferably methyl, ethyl, propyl or butyl, more preferably R 4 is methyl. Preferably, R 3 a...
Embodiment 1
[0280] 2-oxo-3(S)-{3-(pyridin-3-ylmethylsulfonyl)-2(R)-[2,2,2-trifluoro-1(S)-(4-fluorophenyl ) ethylamino]-propionylamino} hexanoic acid cyclopropylamide synthesis
[0281]
[0282] step 1
[0283] Phthaloxaborane (19.4ml, 182mmol) in dichloromethane (15mL) was added dropwise to S-methyl CBS oxazoborane (13ml, 13mmol) and 2 at -78°C over 30min, 2,2,4'-Tetrafluoroacetophenone (18.2ml, 130.13mmol) in dichloromethane. The reaction mixture was stirred overnight at -78°C. The reaction mixture was quenched with 4N HCl (13 mL) in dioxane at -78 °C, warmed to room temperature and the solvent was removed under reduced pressure. Add 10% NaHSO to the concentrate 3 solution (200 mL) and the aqueous layer was extracted with hexane. The organic layer was washed with water and washed with MgSO 4 dry. The solvent was removed under reduced pressure to give 2,2,2-trifluoro-1(R)-(4-fluorophenyl)-ethanol as a colorless oil (90% e.e.).
[0284] step 2
[0285] at 0°C, in N 2 Add NaH (...
Embodiment 2
[0299] 2-Oxo-3(S)-3-[2(R)-(2,2,3,3,3-pentafluoropropylamino)-3-(pyridin-3-ylmethylsulfonyl)propionyl Synthesis of Amino]pentanoic Acid Cyclopropylamide
[0300]
[0301] step 1
[0302] To a solution of 2,2,3,3,3-pentafluoropropan-1-ol (1.5 g, 10.0 mmol) and DIPEA (6.1 ml, 35.0 mmol) in dichloromethane (75 mL) at -78°C was added trifluoro Methanesulfonic anhydride (1.78ml, 10.5mmol). After 2.5 h, S-tritylcysteine was added all at once, and the reaction mixture was stirred at 0° C. for 80 min. The reaction mixture was stirred at room temperature for 18 h and then concentrated on a rotovap. Ethyl acetate was added and the reaction mixture was washed with 1N HCl. The organic layer was dried over magnesium sulfate, filtered and concentrated. The crude product was purified by flash chromatography (3 parts hexane / 1 part ethyl acetate + 1% acetic acid) to give 2(R)-(2,2,3,3,3-pentafluoropropylamino)- 3-Tritylsulfanylpropionic acid (3.29 g).
[0303] step 2
[0304] 2(R)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com