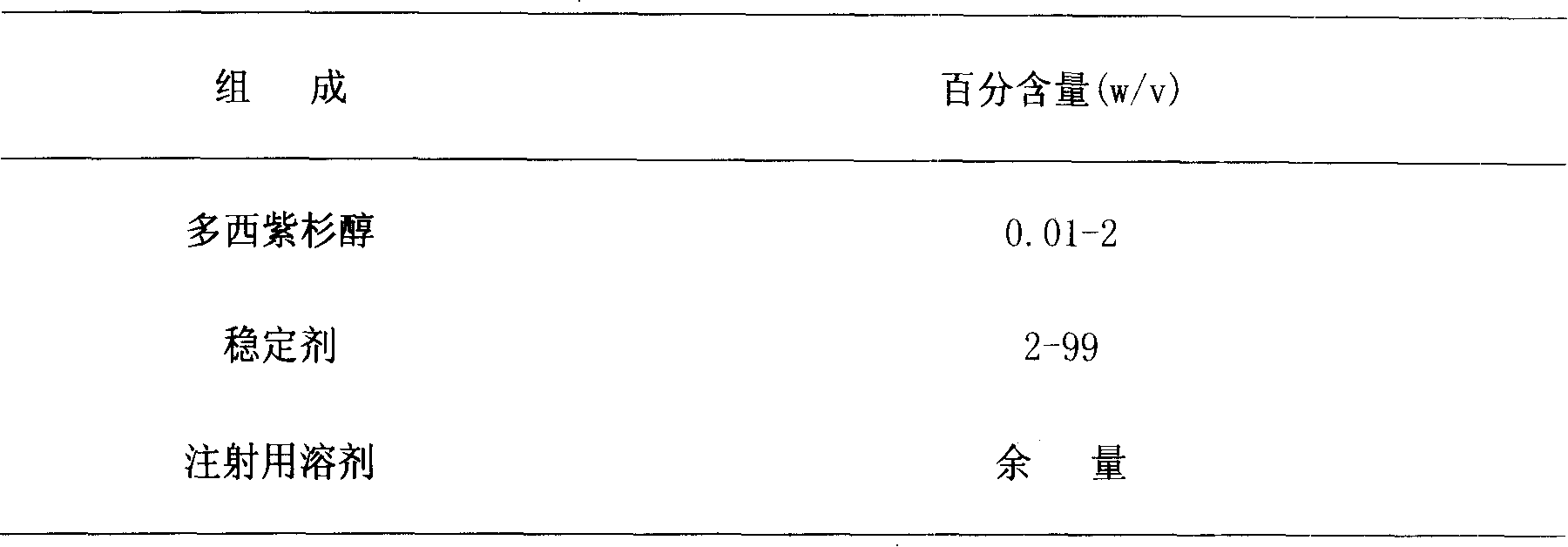
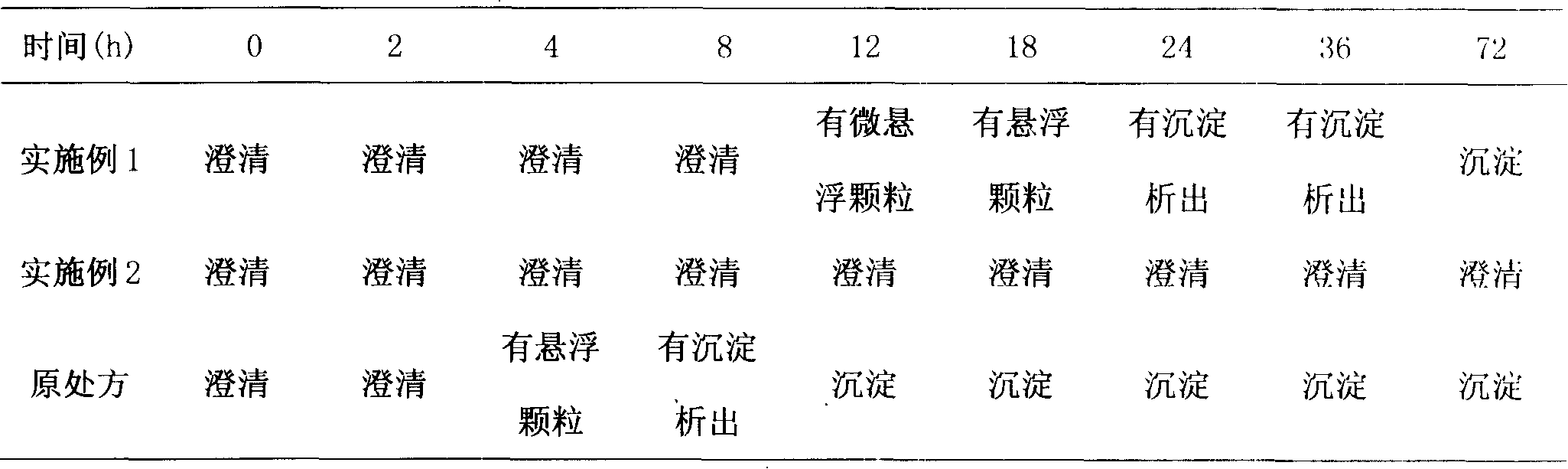
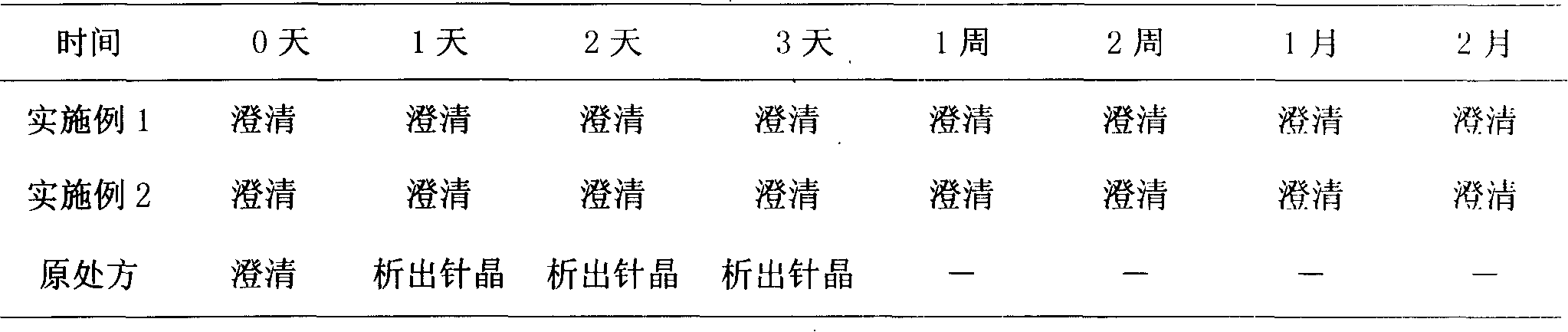
Docetaxel injection and preparation method thereof
A technology for docetaxel and injection, which is applied in the field of novel preparation of docetaxel injection, can solve the problems of limited clinical application and the exertion of good efficacy, complicated preparation method, poor stability and the like, and is suitable for mass preparation and industrialization. The production and preparation methods are simple and easy to implement, and the clinical use is convenient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of Novel Docetaxel Injection
[0031] Weigh 12 grams of soybean lecithin for injection, heat and stir with 30 milliliters of absolute ethanol to dissolve, add 50 grams of Tween-80, mix well, add docetaxel (Shanghai Junjie Biotechnology Co., Ltd., specification: 99.6%, batch No: JJ0512#2) 1000mg, heat and stir to dissolve, add absolute ethanol to 100ml, filter, subpackage, and sterilize.
Embodiment 2
[0032] Example 2 Preparation of Novel Docetaxel Injection
[0033] Weigh 12 grams of soybean lecithin for injection, heat and stir with 30 ml of absolute ethanol to dissolve, add 50 g of Tween-80, mix well, add 600 mg of docetaxel, heat and stir to dissolve, add absolute ethanol to 100 ml, filter , subpackage, and sterilize.
Embodiment 3
[0034] Example 3 Preparation of Novel Docetaxel Injection
[0035] Measure 30 ml of absolute ethanol, add 15 g of soybean lecithin for injection, 1.66 g of cholesterol, 50 g of Tween-80 and stir to dissolve, add 800 mg of docetaxel, stir until dissolved, add absolute ethanol to 100 ml, filter , subpackage, and sterilize.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com