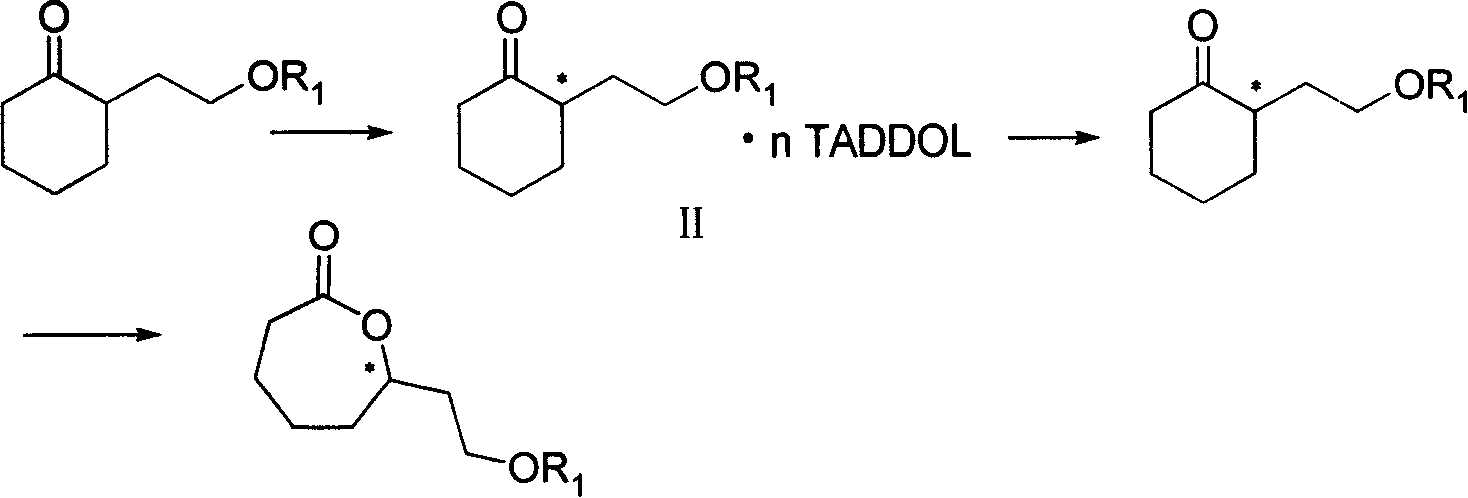
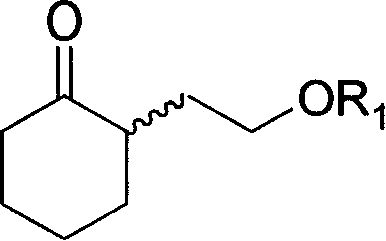
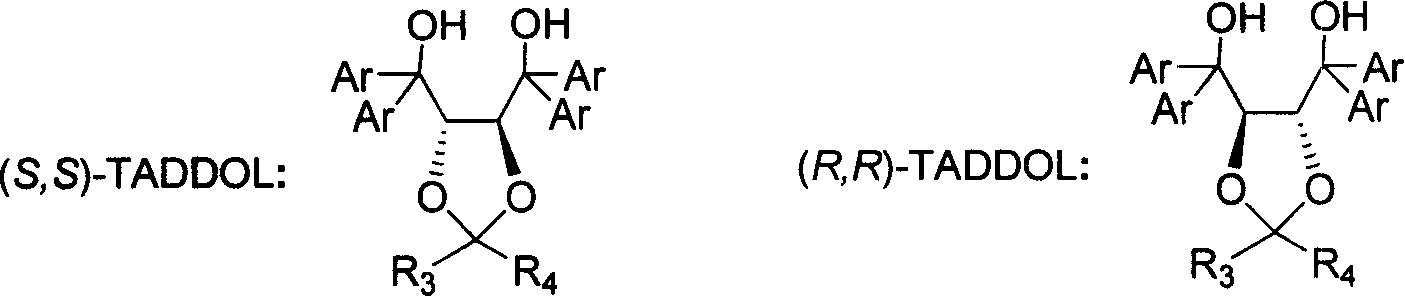(S) or (R)-6-hydroxyl-8-alkoxide octylic acid, its ester, salt, amide, preparation method and application thereof
A technology of alkoxyl and its amide, which is applied in the field of synthesis or -lipoic acid, and can solve the problems of expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~17
[0054] Embodiment 1~17 (S) or (R)-6-hydroxyl-8-alkoxy octanoic acid and its ester
[0055] Table 1 shows examples 1 to 17 of the compound (S) or (R)-6-hydroxy-8-alkoxy octanoic acid and its esters with the structural formula shown in formula I.
[0056] Formula I
[0057] Table 1 (S) or (R)-6-hydroxyl-8-alkoxy octanoic acid and its esters Examples 1-17
[0058] Example
Embodiment 18~22
[0059] Embodiment 18~22 (S) or (R)-6-hydroxyl-8-alkoxy octanoate
[0060] Table 2 shows examples 18-22 of the compound (S) or (R)-6-hydroxy-8-alkoxy octanoate with the structural formula shown in formula II.
[0061] Formula II
[0062] Table 2 (S) or (R)-6-hydroxyl-8-alkoxy octanoate embodiment 18~22
[0063] Example
Embodiment 23~27
[0064] Embodiment 23~27 (S) or (R)-6-hydroxyl-8-alkoxy octanamide
[0065] Table 3 shows examples 23-27 of the compound (S) or (R)-6-hydroxyl-8-alkoxyoctylamide having the structural formula shown in formula III.
[0066] Formula III
[0067] Table 3 (S) or (R)-6-hydroxyl-8-alkoxy octanamide embodiment 23~27
[0068] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com