Technique for drying microorganism medicine sensitive detecting plate
A drying process and detection plate technology, applied in the field of microorganisms, can solve problems such as hole jumping, reduce the degree of antibiotic bacteriostasis, and detachment, and achieve the effects of improving quality, reducing costs, and ensuring accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0072] The specific production process of the drug sensitivity detection plate involved in the present invention is as follows:
[0073] (1) Configure antibiotic diluent
[0074] Add rosemary antioxidants to distilled water to configure an aqueous solution as a dilution of antibiotics;
[0075] (2) Packing
[0076] Dispense the prepared diluent into 15ml per test tube for use;
[0077] (3) Configure stock solution
[0078] Configure the initial stock solution of various antibiotics according to the dilution requirements;
[0079] (4) Dispensing liquid material
[0080] According to the dilution requirements of various slat concentrations, the antibiotic stock solution in step (3) is added to the corresponding subpackaged step (2) test tube.
[0081] (5) Adding samples and packing slats
[0082] Use the dispensing system to add samples to the designated drug-sensitive strips at the corresponding positions of the 96 wells, adding 50ul to each well;
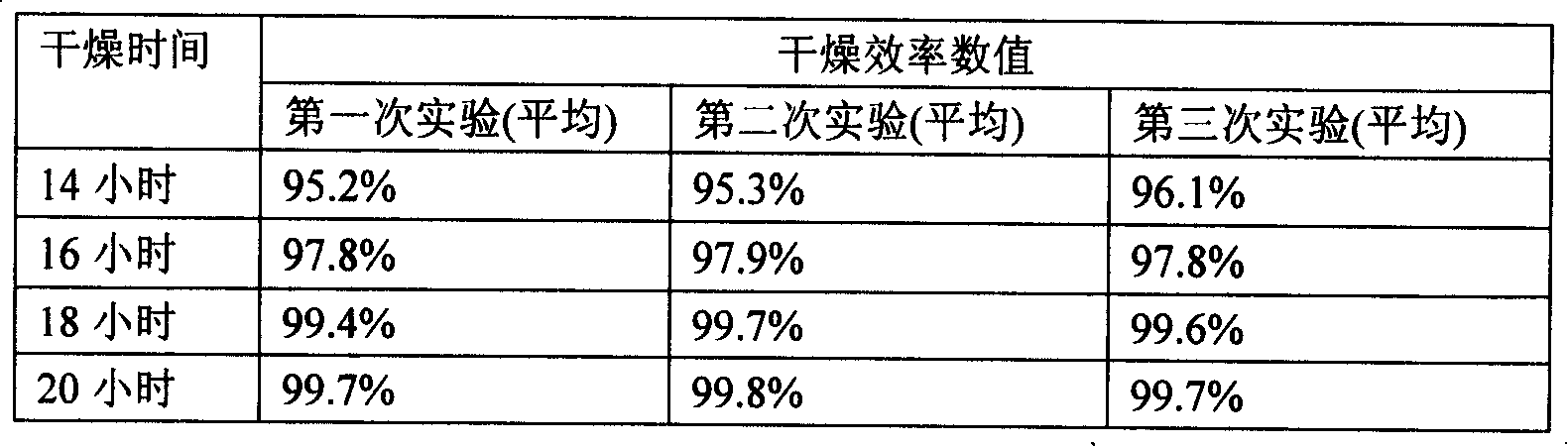
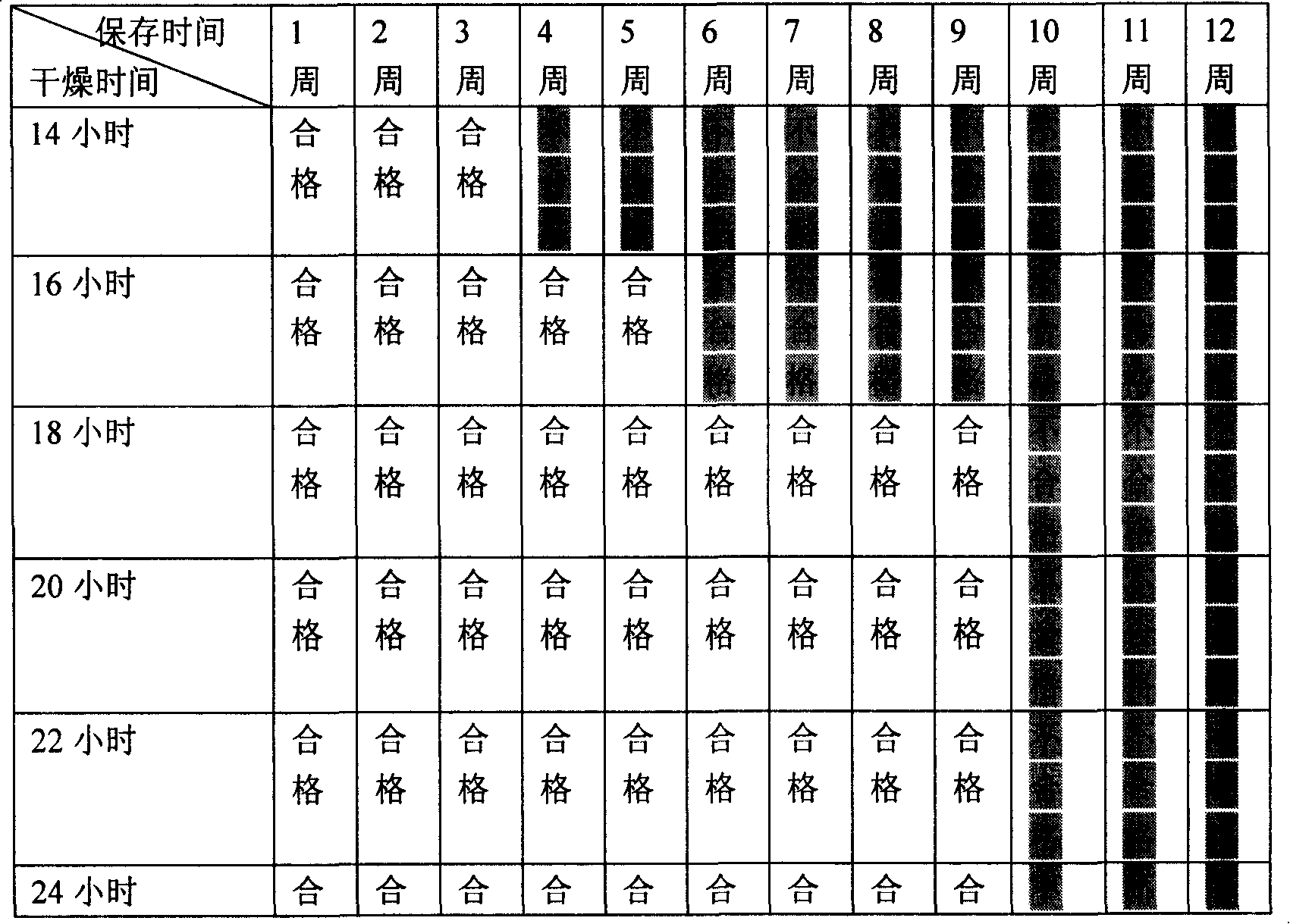
[0083] (6) Slat dryin...
example 2
[0091] The specific production process of the drug sensitivity detection plate involved in the present invention refers to example 1
[0092] Wherein the drying process conditions are:
[0093] ①Ambient temperature: 24°C;
[0094] ② Drying equipment: hair dryer;
[0095] ③Ambient humidity: 34%
[0096] ④ Drying time: 21 hours.
example 3
[0098] The specific production process of the drug sensitivity detection plate involved in the present invention refers to Example 1,
[0099] Wherein the drying process conditions are:
[0100] ①Ambient temperature: 25°C;
[0101] ② Drying equipment: air dryer;
[0102] ③Ambient humidity: 40%
[0103] ④ Drying time: 18 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com