3-amido-2-oxazolidinone derivative hapten and method for preparing same
A technology of oxazolidinone and hapten, which is applied in the field of immunology, can solve the problems of high degree of instrumentation, slow analysis speed, cumbersome methods, etc., and achieve the effects of high titer, easy identification and good specificity
Inactive Publication Date: 2010-11-10
SOUTH CHINA AGRI UNIV
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, these methods are cumbersome, highly instrumented and slow in analysis speed, which cannot meet the timely and rapid detection requirements
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 23
Embodiment 33
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
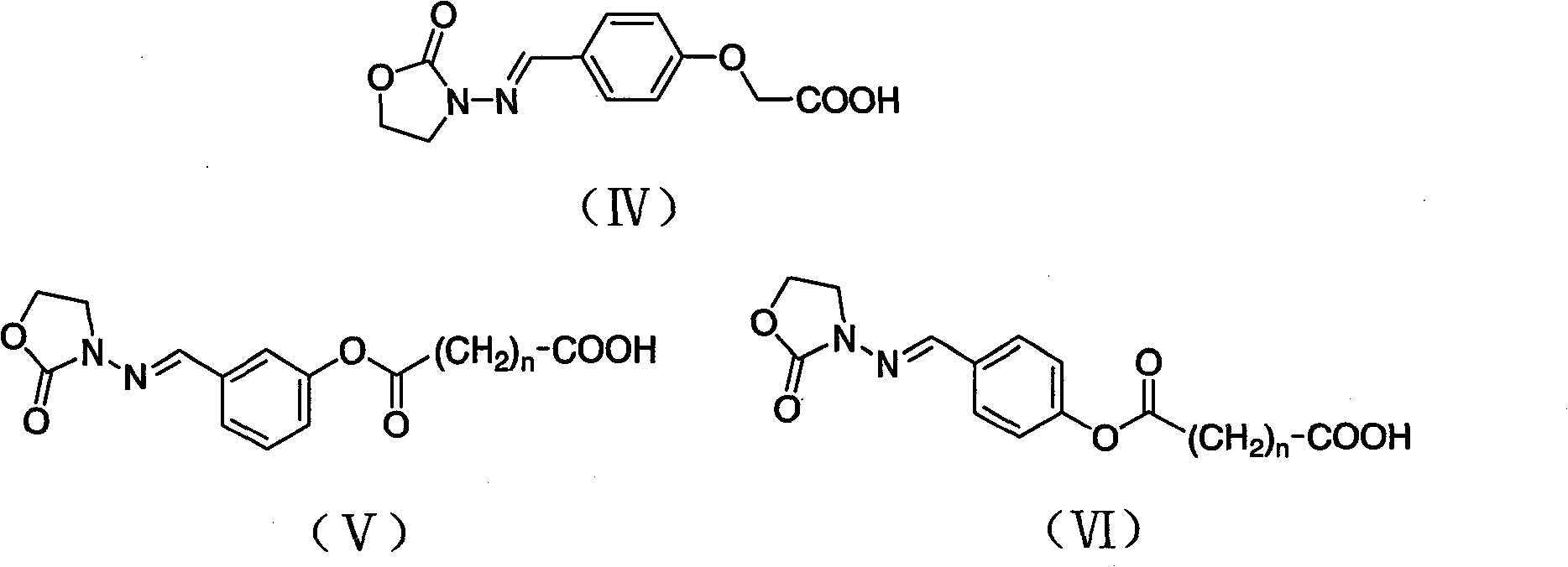
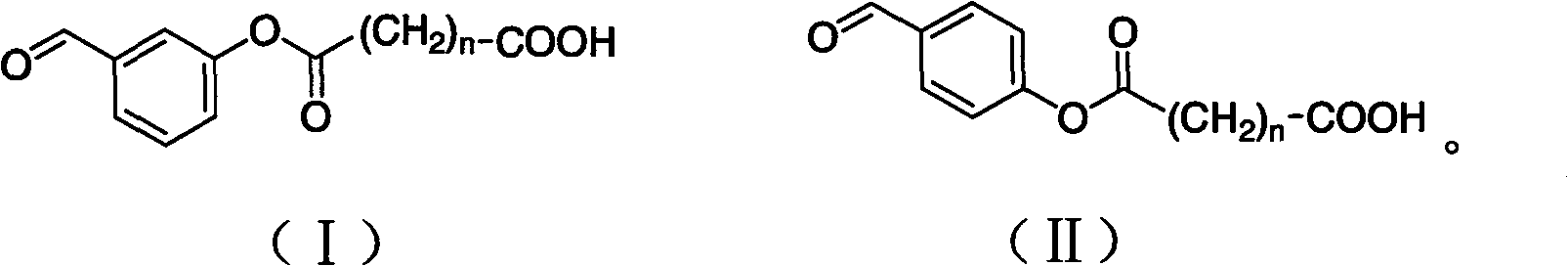
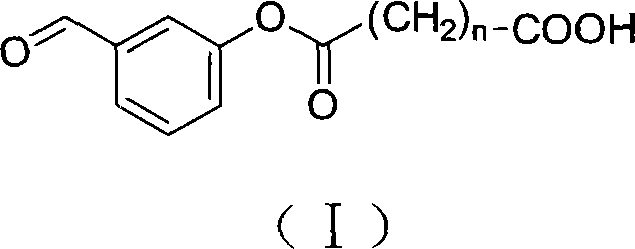
The invention discloses a 3-amino-2-oxazolidone derivative, namely, haptene and the preparation method thereof. The 3-amino-2-oxazolidone derivative haptene is a product of the condensation of the 3-amino-2-oxazolidone and benzaldehyde derivative with carboxyl, namely, ammonia aldehyde. The invention aims at the 3-amino-2-oxazolidone derivative, the haptene is similar to a 3-amino-2-oxazolidone derivative antimer on a molecular structure, stereochemistry and electronic distribution. Spacer arms in the haptene structure are uneasy to induce and generate an arm antibody, a saturated carbon chain with certain length is also used, and thus the organism can be more easily identified. Haptene molecules are provided with active groups convenient to be coupled with a protein carrier, and the existence of the active groups has no influence on the electronic distribution of the molecules of a substance to be measured. After the haptene is coupled with carrier protein, an antibody which has highpotency and good specificity and aims at the 3-amino-2-oxazolidone derivative can be generated by the organism.
Description
3-amino-2-oxazolidinone derivative hapten and its preparation method technical field The invention belongs to the technical field of immunology, and in particular relates to a 3-amino-2-oxazolidinone derivative hapten and its preparation technology. Background technique Furazolidone, also known as furazolidone, is a nitrofuran drug, a broad-spectrum antibiotic, and has certain antibacterial effects on Gram-positive and negative bacteria. Due to its low price and good antibacterial effect, furazolidone is widely used in the prevention and treatment of poultry, livestock, and aquatic dysentery, enteritis, coccidiosis, and turkey blackhead. However, relevant studies have proved that nitrofuran drugs and their metabolites have considerable toxicity, teratogenic side effects, and can induce cancer, which has attracted people's attention. In July 1990, the European Union promulgated Regulation 2377 / 90 / EEC, which listed nitrofuran drugs and their metabolites as Class A prohibite...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D263/26G01N33/53
Inventor 沈玉栋张世伟孙远明肖治理雷红涛王弘
Owner SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com