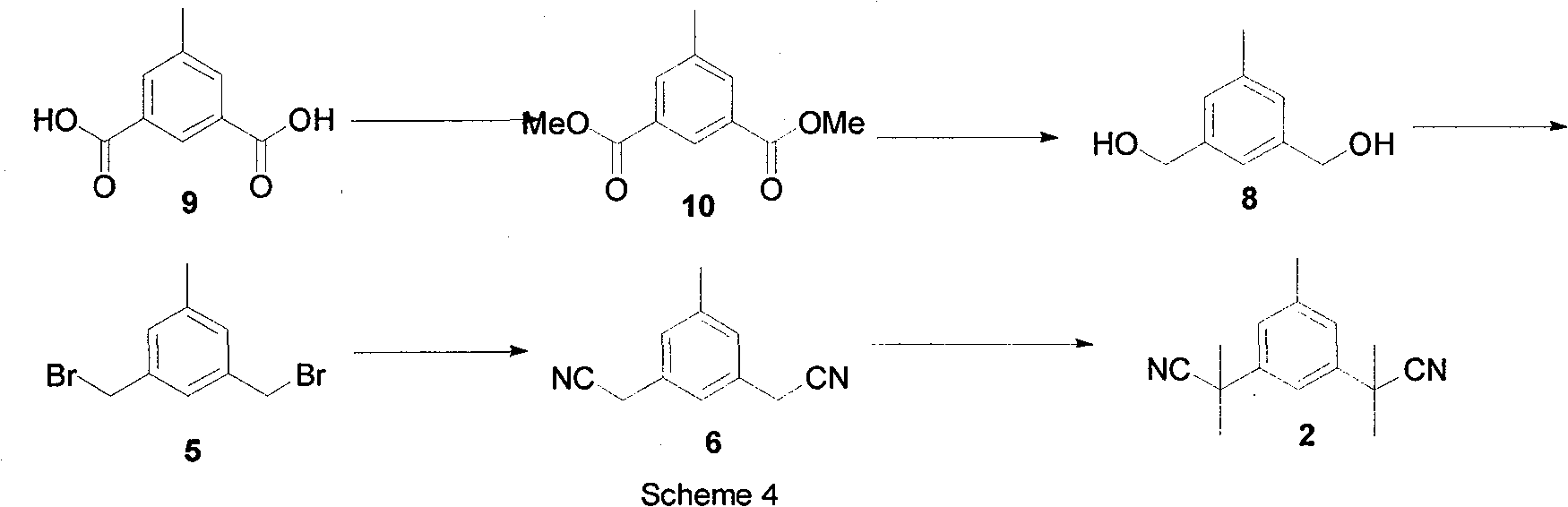
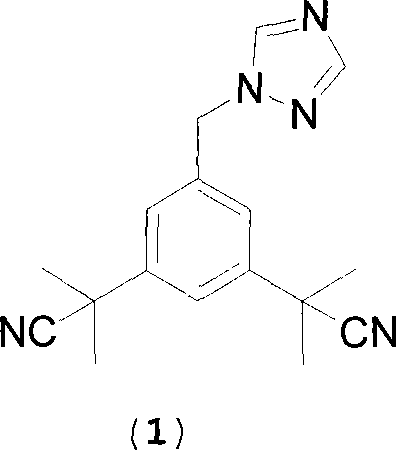
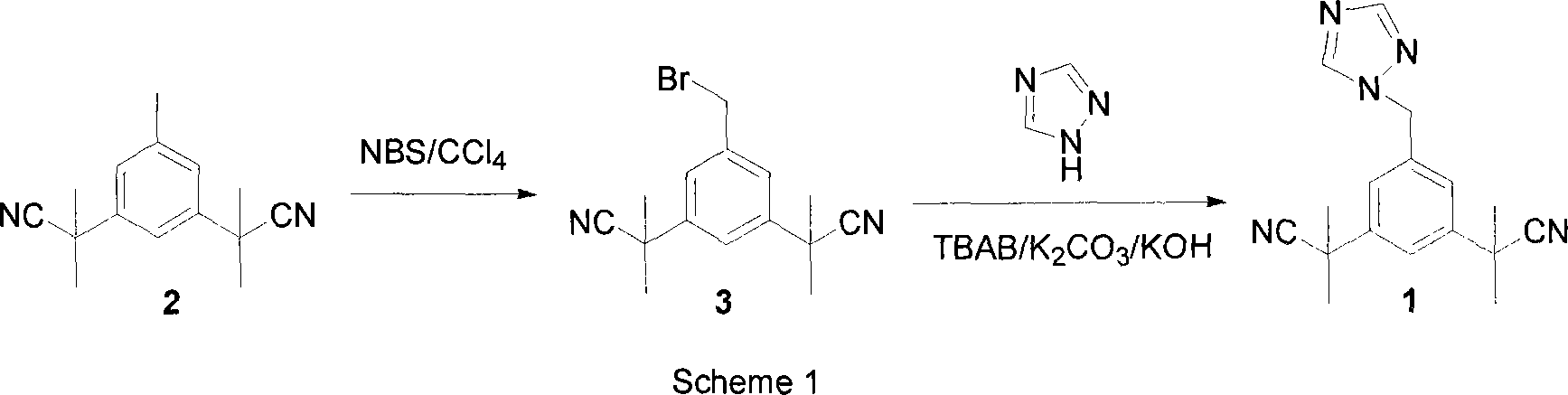
Preparation of 3,5-di(2-cyano-isopropyl)-toluene
A technology of isopropyl and toluene, applied in the field of pharmaceutical intermediates 3, can solve the problems of low yield and purity, high price, high toxicity of carbon tetrachloride, etc., and achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] Step 1: Synthesis of compound (10)
[0023] Add 400 mL methanol, 8 mL concentrated sulfuric acid, and 180 g (1 mol) 5-methyl iso-dibenzoic acid into a 1 L three-necked flask, and heat to reflux for 16 hours. Stop the reaction, add 400mL Na after cooling to room temperature 2 CO 3 Solution. A solid precipitated out. After suction filtration and drying, 185.6 g of compound 10 was obtained, and the yield was 90%. mp: 960°C-980°C. 1 H NMR(500MHz, CDCl 3 ): δ = 2.45 (s, 3H), 3.99 (s, 6H), 8.04 (s, 2H), 8.48 (s, 1H), 13 C NMR(100MHz, CDCl 3 ): δ=21.1, 52.3, 127.9, 130.5, 134.4, 138.6, 166.4. MS(EI): m / z=208
[0024] Step 2: Synthesis of compound (8)
[0025] Add 20.8g (0.1mol) compound (10), 150mL tetrahydrofuran, 37.1g (0.7mol) KBH into a 500mL three-necked flask 4 , 29.7g (0.7mol) LiCl, heated to reflux for 5-7 hours. Then stop heating, cool to room temperature, and slowly add 400mL saturated NH 4 The Cl solution was stirred for 1 hour, and the insoluble matter was removed by s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com