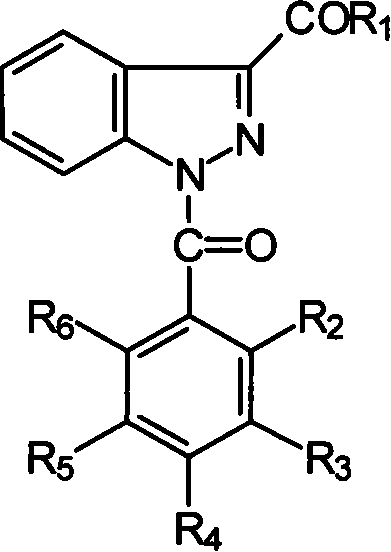
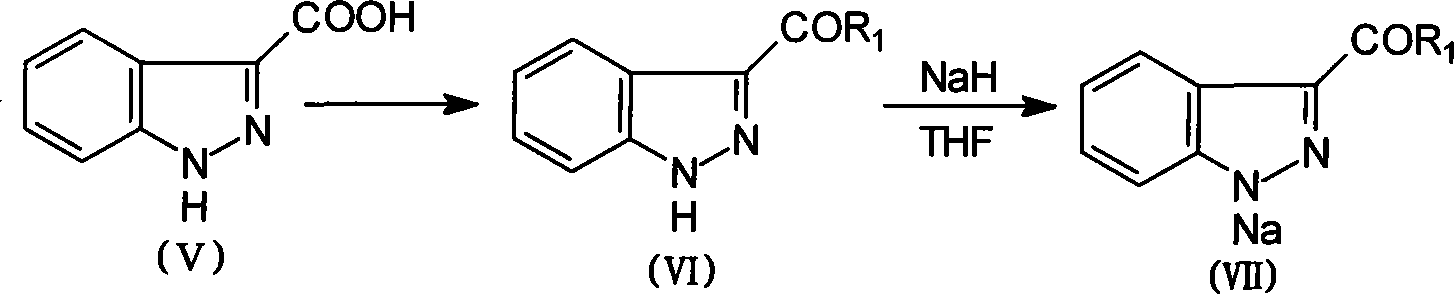
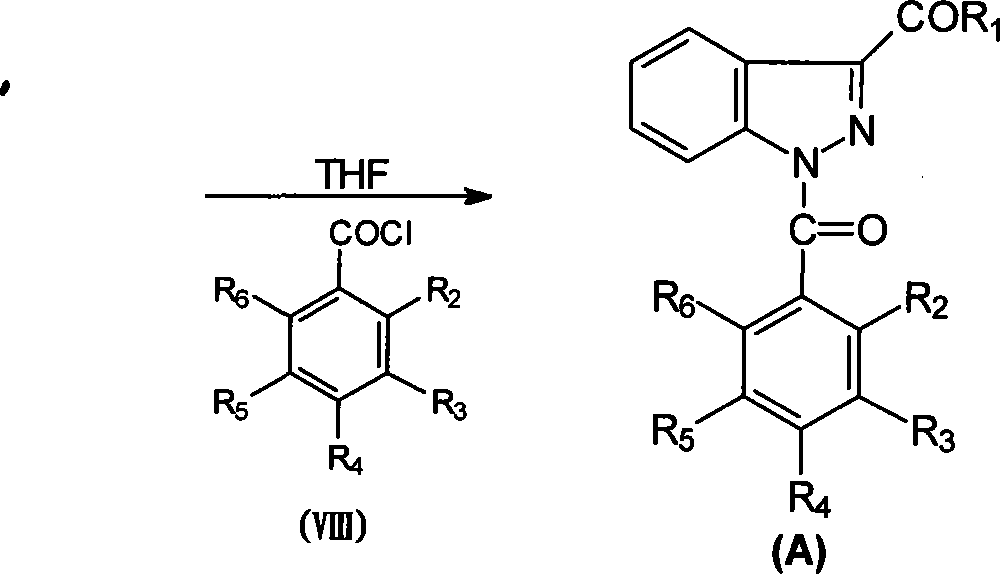
1-(substituted benzoyl)-indazole-3-carboxylate or amides compounds thereof, synthesis and application thereof
A kind of amide compound, benzoyl technology, applied in the field of pharmaceutical compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1. Preparation of N-acetophenazine (Compound II)
[0088] Take 108.14g (1mol) of phenylhydrazine, 200ml of water, 55.9g (0.931mol) of acetic acid, stir, heat to reflux for 5-7 hours, add, let cool, slowly precipitate a solid, filter, and recrystallize the solid with water to obtain the compound II 115g, yield 76.6%, mp: 128-130°C.
Embodiment 2
[0089] Example 2. Preparation of N-Acetylaminoisonitrosoacetanilide (Compound III)
[0090] Take compound II 75.1g (0.50mol), hydroxylamine hydrochloride 104.2g (1.5mol), sodium sulfate 500g and water 1650ml solution, 1N hydrochloric acid 250ml stir, heat to 95 ℃, add chloral hydrate 107.5g (0.65mol) within 30 minutes ) A solution of 220ml of water, stir and react for 3 hours after the addition is complete, and after a little cooling, add 5-10g of activated carbon, then heat to 100°C, and filter while hot. The filtrate was allowed to cool, crystals were slowly precipitated, filtered, the solid was washed with an appropriate amount of water, drained, and dried to obtain 86 g of compound (III) with a yield of 77.8%, mp: 132-135°C.
Embodiment 3、1
[0091] Example 3. Preparation of 1H-indazole-3-carboxylic acid (Intermediate V)
[0092] Compound III 55.3g (0.25mol) was partially added to 250ml of 98% sulfuric acid at 55°C. After the addition, the reaction temperature was raised to 85°C, stirred and reacted for 15 minutes. After cooling, the reactants were slowly poured Pour into 700g of crushed ice, reflux the resulting suspension for 3 hours, let cool, precipitate crystals, filter, wash with water, and dry to obtain a crude product of 42g. The crude product is recrystallized with glacial acetic acid to obtain compound V29g, the yield is 71.5%, mp: 265~267℃ . [NMR H spectrum: 7.33 (q, 1H), 8.36 (s, 1H), 8.37 (t, 1H), 8.45 (t, 1H), 10.07 (broad peak, N-H), 12.76 (s, -COOH)]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com