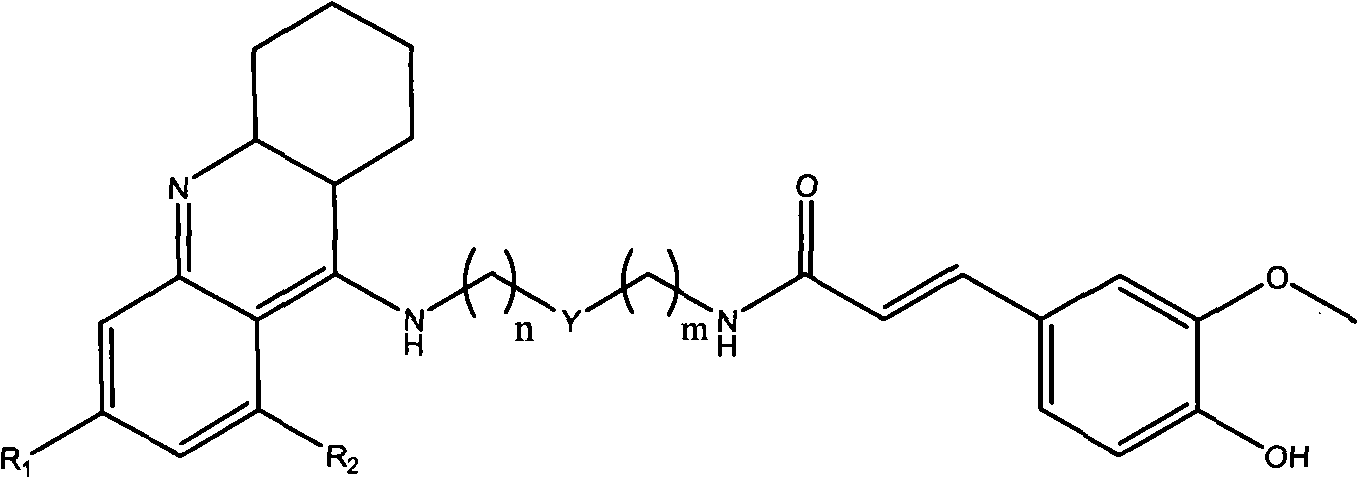
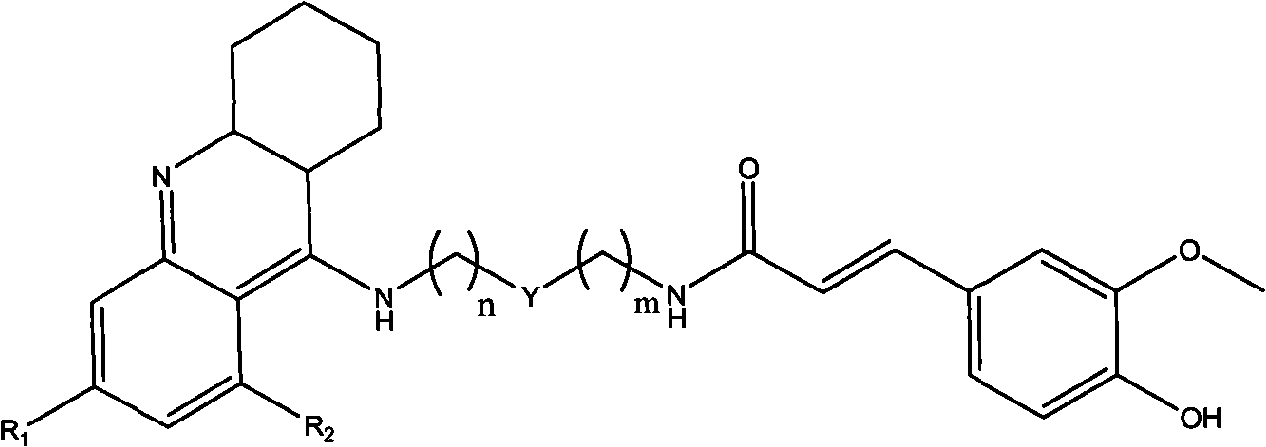
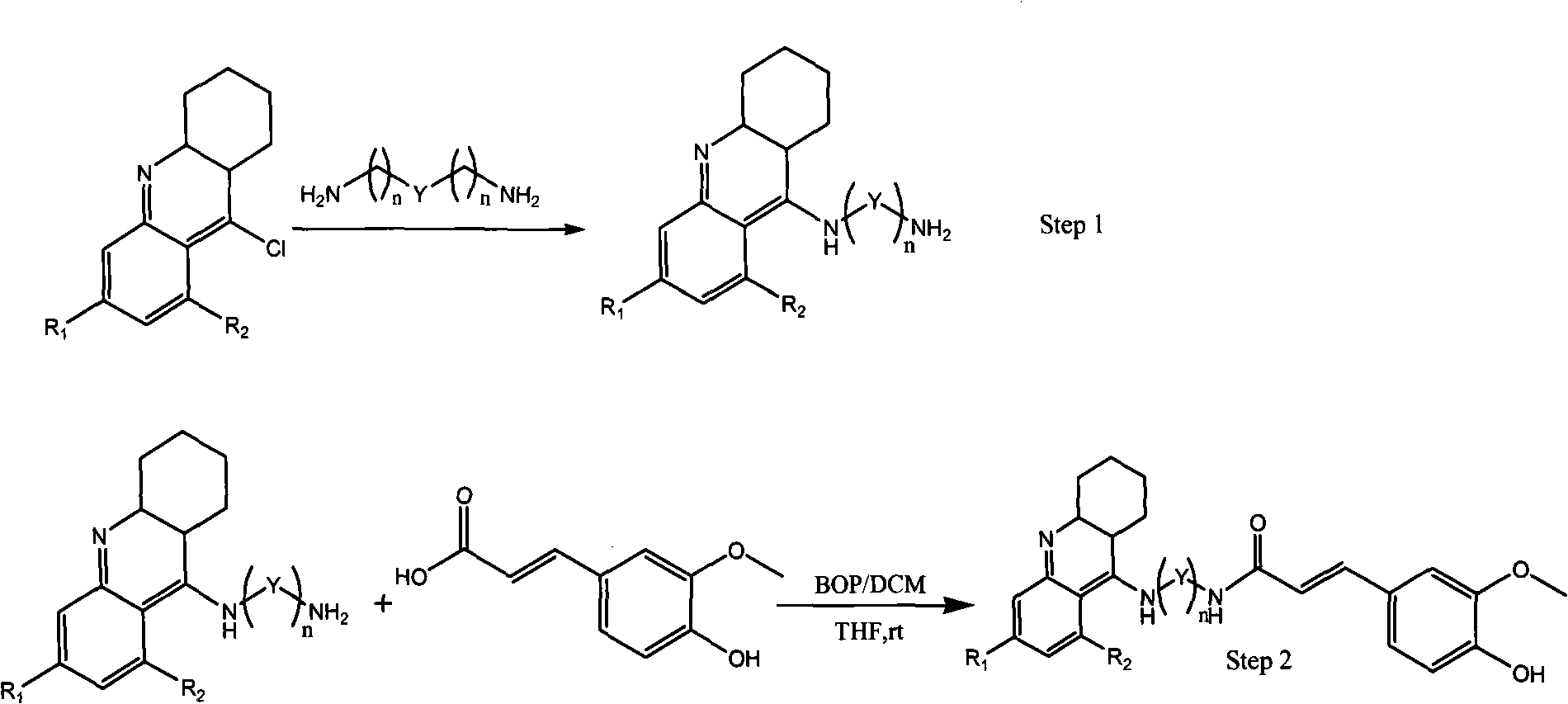
Tacrine-ferulaic acid hetero-blend, preparation method and pharmaceutical compositions thereof
A technology for drugs and compounds, applied in the field of medicine, can solve the problems of elevated transaminases, frequent oral administration, water solubility and poor human tolerance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] 3-Methoxy-4-hydroxy-ferulic acid-{N-(2-[2-(1,2,3,4-tetrahydro-acridin-9-amino)-ethyl]-methyl -amino]}-amide.
[0046] Reagents: Ferulic acid (170mg, 8.3mmol), anhydrous THF (35ml), BOP (770mg, 17mmol), and {N-(1,2,3,4-tetrahydro-acridin-9-amino) -N'-methyl}-1.2-bisethylenediamine (200 mg, 6.7 mmol).
[0047] Method: Dissolve ferulic acid in anhydrous THF, add triethylamine, stir at room temperature, then add BOP dropwise, and finally add {N-(1,2,3,4-tetrahydro-acridine-9-amino )-N'-methyl}-1.2-bisethylenediamine, stirred overnight at room temperature. After concentration, it was directly separated by silica gel column chromatography, eluent: dichloromethane:methanol=11:3.
[0048] Purification: silica gel column chromatography using DCM / MeOH (12:1). White solid, yield: 110 mg (35%)
[0049] 1 H NMR results are:
[0050] 1 HNMR (DMSO, 400MHz, δppm): 9.38(s, 1H), 7.90-7.84(m, 2H), 7.80(m, 1H), 7.78-7.77(m, 1H), 7.60-7.56(m, 1H), 7.30-7.56(d, 1H), 7.09...
Embodiment 2
[0054]
[0055] 3-Methoxy-4-hydroxy-ferulic acid-{N-(2-[2-(6-chloro-1,2,3,4-tetrahydro-acridine-9-amino)-ethyl ]-methyl-amino]}-amide.
[0056] Reagents: Ferulic acid (170 mg, 8.3 mmol), anhydrous THF (35 ml), BOP (770 mg, 17 mmol), and {N-(6-chloro-1,2,3,4-tetrahydro-acridine-9 -amino)-N'-methyl}-1.2-diethylenediamine (200 mg, 6.0 mmol).
[0057] Purification: silica gel column chromatography using dichloromethane:methanol DCM / MeOH (12:1). White solid, yield: 106 mg (34%).
[0058] Except that the reagents and purification columns are different, the rest of the preparation and purification steps are the same as in Example 1.
[0059] 1 HNMR (DMSO, 400MHz, δppm): 9.38(s, 1H), 7.90-7.84(m, 1H), 7.80(m, 1H), 7.78-7.77(m, 1H), 7.60-7.56(m, 1H), 7.30-7.56(d, 1H), 7.09(d, 1H), 6.97-6.95(d, 1H), 6.79-6.77(d, 1H), .6.42-6.39(d, 1H), 3.73(s, 6H) , 3.63-3.60 (m, 2H), 3.34-3.30 (m, 2H), 2.97 (br, 2H), 2.65 (br, 2H), 1.83 (br, 4H), 1.75-1.31 (m, 4H).
[0060] 13 C NMR (DMSO, 1...
Embodiment 3
[0062]
[0063] 3-Methoxy-4-hydroxy-ferulic acid-{N-[(1,2,3,4-tetrahydro-acridin-9-amino)-pentyl-3-one]}-amide
[0064] Reagents: Ferulic acid (170mg, 8.3mmol), anhydrous THF (35ml), BOP (770mg, 17mmol), and {N-[(1,2,3,4-tetrahydro-acridin-9-amino )-3-keto]}-pentamethylenediamine (200 mg, 6.1 mmol).
[0065] Purification: silica gel column chromatography using DCM / MeOH (12:1). Yellow solid, yield: 105 mg (30%).
[0066] Except that the reagents and purification columns are different, the rest of the preparation and purification steps are the same as in Example 1.
[0067] 1HNMR (DMSO, 400MHz, δppm): 9.38(s, 1H), 7.90-7.84(m, 2H), 7.80(m, 1H), 7.78-7.77(m, 1H), 7.60-7.56(m, 1H), 7.30-7.56(d, 1H), 7.09(d, 1H), 6.97-6.95(d, 1H), 6.79-6.77(d, 1H), .6.42-6.39(d, 1H), 3.73(s, 3H) , 3.63-3.60 (m, 2H), 3.34-3.30 (m, 2H), 2.97 (br, 2H), 2.65 (br, 2H), 1.83 (br, 4H), 1.60-1.41 (m, 4H).
[0068] 13 C NMR (DMSO, 100MHz, δppm): δ210.8, 168.8, 161.4, 158.3, 150.1, 151.2, 148.2, 14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com