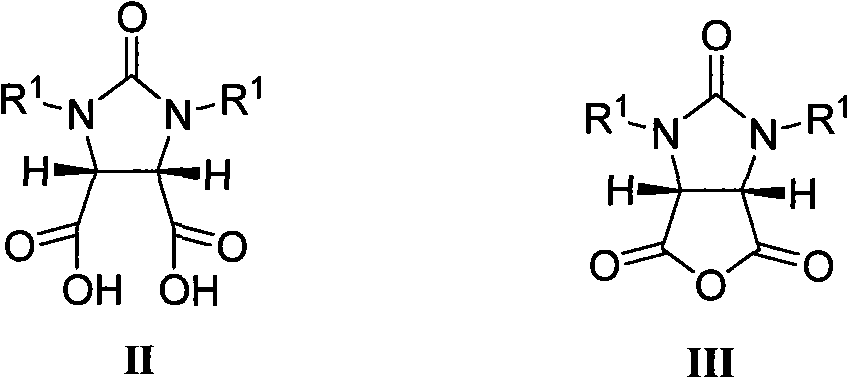Stereoselective total synthesis method of (+)-biotin
A technology of stereoselectivity and biotin, applied in the direction of organic chemistry, can solve the problems of cumbersome operation and application limitations, and achieve the effect of mild reaction conditions, easy operation and good stability
Inactive Publication Date: 2008-10-15
FUDAN UNIV
View PDF7 Cites 40 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
These methods all have the disadvantage of cumbersome operation, which limits their application.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
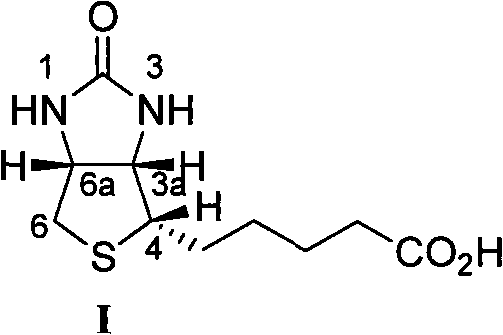
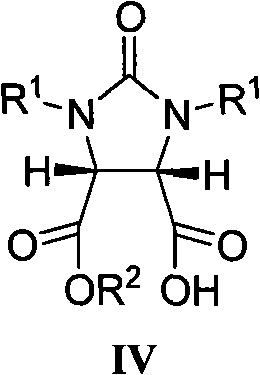
The invention belongs to the organic chemistry technical field, in particular to a (+) - biotin stereo-selective full synthesis method. The synthesis method comprises the following steps of preparing meso cyclic anhydride via ring-closing reaction and acyl halide dehydration with diacid as initial materials; preparing (4S, 5R)-half ester under the presence of cinchona alkaloid via enantioselective ring opening; then producing intermediate product (3aS,6aR)-lactone, (3aS,6aR)-thiolactone, (3aS,4S,6aR)-double-benzyl biotin and the derivative of the same via selective reduction, acid promoted cyclization, Fukuyama and coupling reaction and so on; debenzylating and ring opening upon the function of inorganic acid, and producing (+)- biotin via cycization reaction in inorganic base solution upon catalysis of activated carbon. With the synthesis method, raw materials are readily available, reaction conditions are mild and operation is easy; the catalyst used has good stability, is easy to be recycled and is suitable for industrial production.
Description
Stereoselective Total Synthesis of (+)-Biotin technical field The invention belongs to the technical field of organic chemistry, and in particular relates to a stereoselective total synthesis method of natural product (+)-biotin. Background technique (+)-biotin ((+)-biotin) I, also known as vitamin H or coenzyme R, is a water-soluble B vitamin. As a prosthetic group of various carboxylases, (+)-biotin plays an important role in the formation of reversible carboxyl groups and the transfer of CO2, and it is needed for the growth of the entire biological world. Its structural formula is as shown in formula I: Harris et al reported the total synthetic route of (±)-biotin for the first time in 1944 (J.Am.Chem.Soc.1944,66,1756.), this route is starting material with L-cystine, through Reduction, coupling, Claisen condensation and more than 13 steps were performed to obtain (±)-biotin with a total yield of about 10%. So far, there have been many total synthetic routes repor...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D495/04
Inventor 陈芬儿戴惠芳黄建熊非
Owner FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com