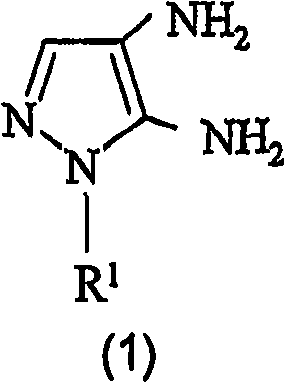
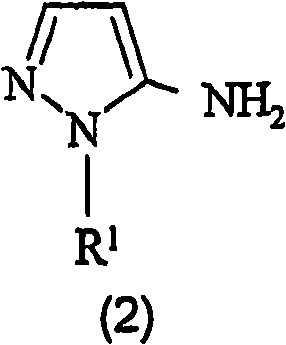
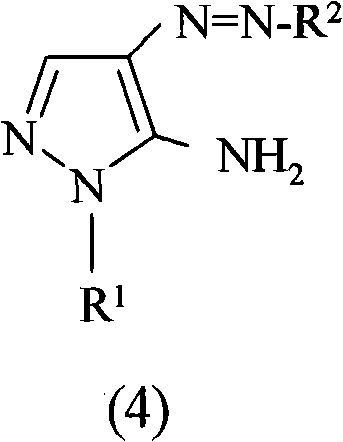
Preparation of 4,5-diamino-1-(substituted)-pyrazole and acid addition salts thereof
A technology for acid addition salts and pyrazoles, which is applied in the fields of preparing 4, preparing 5-amino-1-pyrazoles, sulfuric acid addition salts, and azo compounds, can solve problems such as inability to obtain separated products, and achieves safe operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0044] The method provided by the present invention is further illustrated by the following examples. All percentages in the examples are % by weight unless otherwise stated.
[0045] 5-Amino-4-carboxy-1-(2’-hydroxyethyl)-pyrazole hydrochloride
[0046] Ethanol (80ml) and 2-hydroxyethylhydrazine (245.04g, 90% analytical grade) were mixed in a stirred round bottom flask. A solution of ethyl (ethoxymethylene)cyanoacetate (470.4 g) in ethanol (300 mL) was added causing the reaction mixture to exotherm to 75-80 °C. The mixture was maintained at 75-80°C for 3 hours and then cooled to room temperature. A 50% aqueous sodium hydroxide solution (282.2 g) was diluted with water (94 mL) and added to the reaction mixture. The resulting mixture was heated to 75-80°C for 3 hours. Water (300 mL) was added and the solution was cooled to 10-15°C. The hydrochloric acid addition salt of 5-amino-4-carboxy-1-(2'-hydroxyethyl)-pyrazole was obtained by precipitation by addition of aqueous hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com