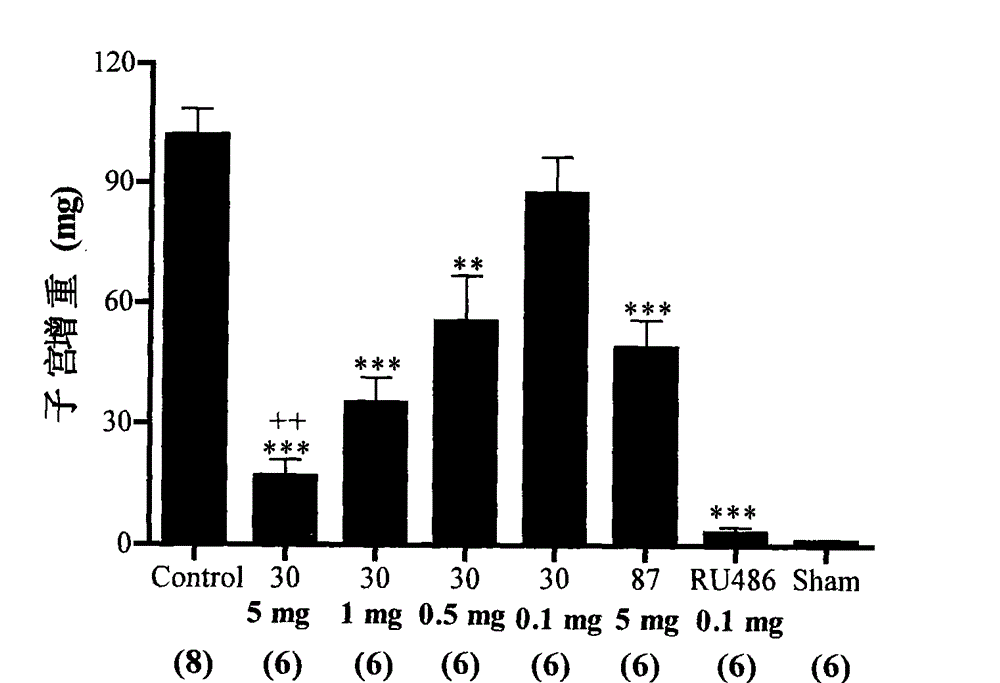
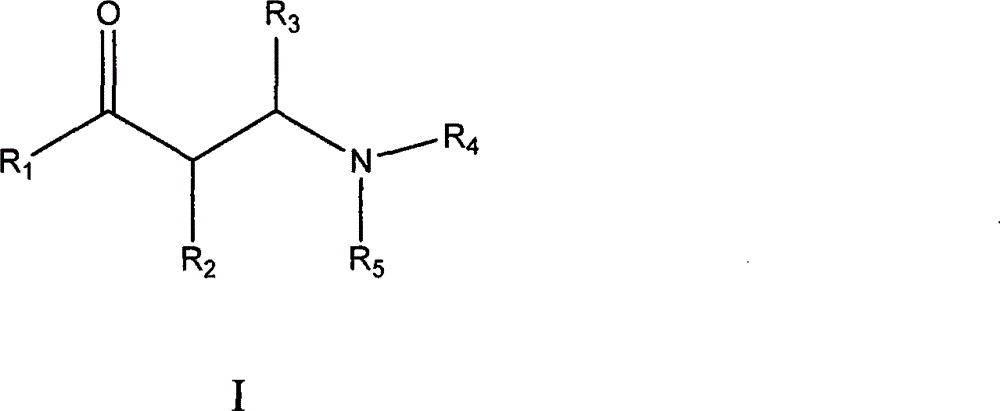
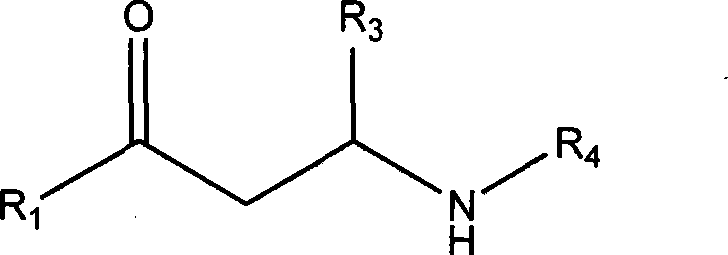
Non-steroid progestogen receptor regulator, preparation thereof, pharmaceutical compositions and uses
A progesterone receptor, non-steroidal technology, applied in the field of medicinal chemistry, can solve the problems of poor prognosis of breast cancer and reduce the adhesion of breast cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Synthesis of 3-cyclohexyl-3-(4-nitroanilino)-1-(4-methylphenyl)-1-propanone
[0073] Specific preparation method: dissolve 1 mmol of 4-nitroaniline in 1 to 3 ml of absolute ethanol, add 1 mmol of cyclohexanal and 1 mmol of 4-methylacetophenone, stir and dissolve at room temperature, and add 20 to 100 μL (catalytic amount) of concentrated hydrochloric acid or saturated ethanol solution of hydrochloric acid, precipitation occurs, stir at 0°C to 55°C for 8 to 48 hours, place in the refrigerator overnight, filter with suction, wash the solid with a small amount of ethanol to obtain a crude product. Pure product can be obtained by recrystallization with ethanol or acetone. The yield is 50%~99%.
[0074] 1 HNMR (DMSO-d 6 , 400MHz): δ7.95 (2H, d, J = 8.5), 7.87 (2H, d, J = 8.1), 7.33 (2H, d, J = 8.2), 7.15 (1H, d, J = 9.2, NH ), 6.64 (2H, d, J=9.5), 4.06 (1H, d, J=8.6), 3.24 (2H, t), 2.07 (3H, s), 1.54~1.80 (6H, m), 1.03~1.21 (5H, m); orange solid with a melting point of ...
Embodiment 2
[0076] Synthesis of 3-cyclohexyl-3-(4-nitroanilino)-1-(4-bromophenyl)-1-propanone
[0077] Specific preparation method: prepare according to the same method as in Example 1 except that 4-bromoacetophenone is used instead of 4-methylacetophenone.
[0078] 1 HNMR (DMSO-d 6 , 400MHz): δ7.95 (2H, d, J=8.7), 7.90 (2H, d, J=8.7), 7.73 (2H, d, J=8.3), 6.64 (2H, d, J=9.1), 4.02 (1H, q, J=11.7, 5.9), 3.26 (2H, d, J=6.3), 1.56~1.79 (6H, m), 1.03~1.20 (5H, m); yellow solid, melting point: 161~ 162°C (159°C).
Embodiment 3
[0080] Synthesis of 3-cyclohexyl-3-(4-nitroanilino)-1-(3-fluorophenyl)-1-propanone
[0081] Specific preparation method: except that 4-methylacetophenone was replaced with 3-fluoroacetophenone, the preparation was carried out in the same manner as in Example 1.
[0082] 1 HNMR (DMSO-d 6 , 400MHz): δ7.95(2H, d, J=8.4), 7.83(1H, d, J=7.3), 7.75(1H, d, J=9.2), 7.48~7.62(2H, m), 7.13( d, NH), 6.65 (2H, d, J=9.0), 4.05 (1H, d, J=4.4), 3.30 (2H, d, J=5.9), 1.60~1.80 (6H, m), 1.06~1.22 (5H, m); orange solid, melting point: 131-133°C (105°C).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com