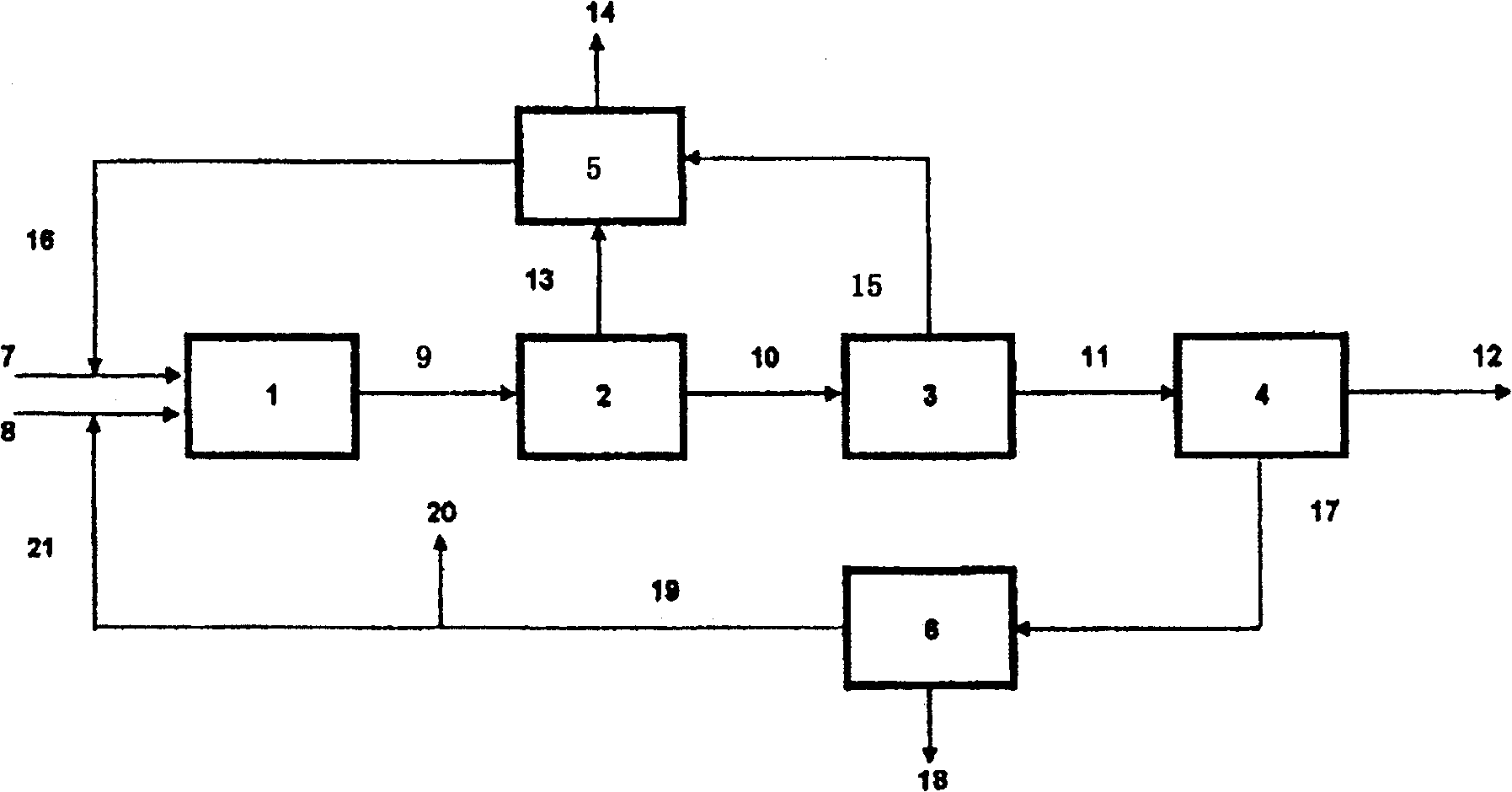
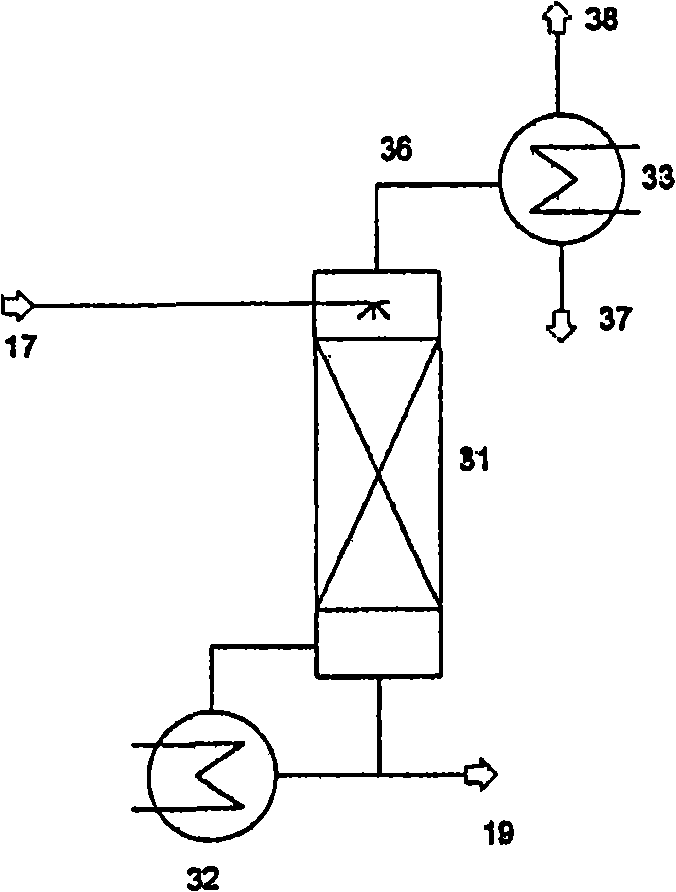
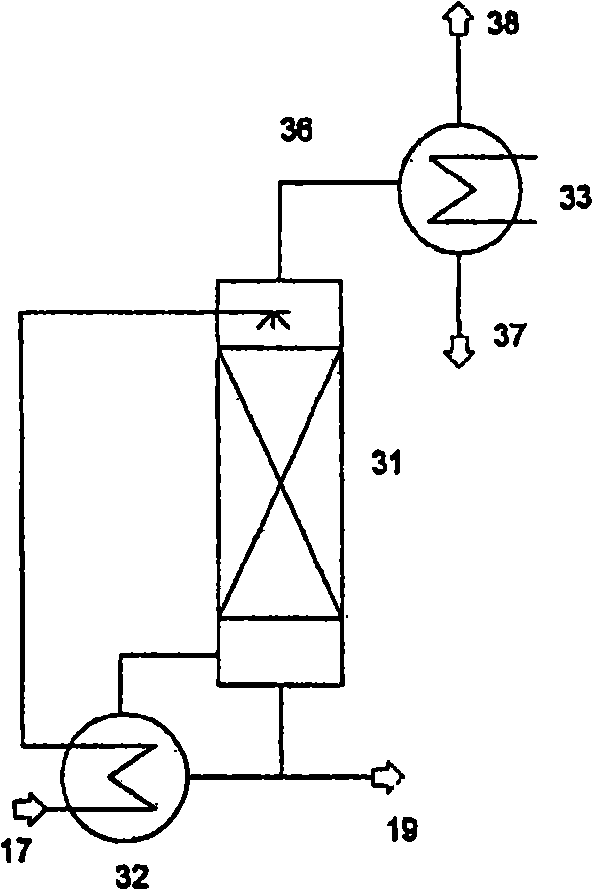
Method for producing isocyanate
A technology of isocyanate and crude isocyanate, which is applied in the field of isocyanate production, can solve the problems of not describing the purity of circulating solvents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 (Preparation of a mixture of diamine and polyamine)
[0035] In a stirred container, 2600 grams of aniline and 1000 grams of formalin (based on the weight of the solution, 30% by weight aqueous formaldehyde solution) are thoroughly mixed under stirring and at 25°C, during which time the mixture is heated To 60°C. Turn off the stirrer, and separate and remove the water phase stabilized on the upper part. Then, 68 g of a 30% by weight hydrochloric acid aqueous solution was mixed in, while stirring and cooling, and the temperature was maintained at 45°C. Stirring was continued at this temperature for 15 minutes, and heating was used instead of cooling. The mixture was uniformly heated to 140°C under a pressure of 5 bar within 120 minutes, and maintained at this temperature for 15 minutes.
[0036] Then, the mixture was cooled to 100°C, reduced to normal pressure, and 54 g of a 50% by weight aqueous sodium hydroxide solution was added for neutralization while stirri...
Embodiment 2
[0042] Example 2 (Use contaminated solvent to produce a mixture of diisocyanate and polyisocyanate (not according to the invention))
[0043] In a stirred reactor, the mixture of 1900 g of diamine and polyamine obtained in Example 1 was dissolved in 5700 g of chlorobenzene. The phosgene content was 200 ppm based on the weight of the solvent chlorobenzene. MDI The content is 200ppm. In a second container made of stainless steel (DIN 1.4571), a 33% by weight (based on the weight of the solution) phosgene solution was prepared by dissolving 3800 grams of phosgene in 7600 grams of chlorobenzene, while cooling to At 0°C, mix the amine with the phosgene solution while stirring vigorously. The resulting solid suspension is then slowly heated to form hydrogen chloride gas, which is collected by a suitable device. During this process, a homogeneous polyisocyanate solution is formed. The solvent was separated and removed by distillation, and as a result, a mixture of 2370 grams of diisocy...
Embodiment 3
[0051] Example 3 (Use pure solvent to produce a mixture of diisocyanate and polyisocyanate (according to the present invention))
[0052] In a stirred reactor, the mixture of 1900 g of diamine and polyamine obtained in Example 1 was dissolved in 5700 g of chlorobenzene. Based on the weight of the solvent chlorobenzene, the phosgene content was 20 ppm, and MDI The content is 20 ppm. In a second container made of stainless steel (DIN 1.4571), a 33% by weight (based on the weight of the solution) phosgene solution was prepared by dissolving 3800 grams of phosgene in 7600 grams of chlorobenzene, while cooling to At 0°C, while stirring vigorously, mix the amine with the phosgene solution. The resulting solid suspension is then slowly heated to form hydrogen chloride gas, which is collected by a suitable device. During this process, a homogeneous polyisocyanate solution is formed. The solvent was removed by distillation, and as a result, a mixture of 2370 grams of diisocyanate and poly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com