2-aminobenzamide derivative
A technology of aminobenzamide and derivatives, applied in the field of 2-aminobenzamide derivatives, can solve the problems of no VR1 receptor record pain, no disclosure, angiogenesis obstruction, etc., and achieve good pharmacological effects
Inactive Publication Date: 2008-11-26
ASTELLAS PHARMA INC
View PDF13 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, each document discloses the inhibition of angiogenesis due to VEGF inhibition, etc., but there is no description about the VR1 receptor and no description about the application to pain.
In addition, none of the documents disclose a compound in which the amino group at the 2-position is directly bonded to a monocyclic ring without passing through an alkylene chain.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
reference example 1
reference example 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
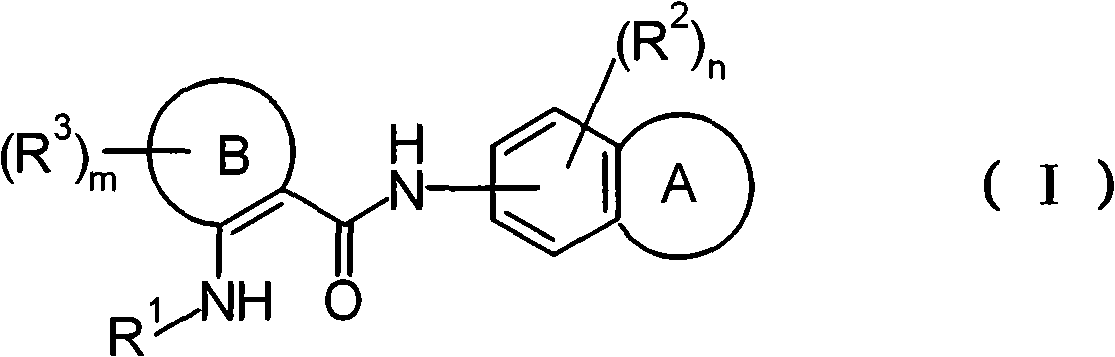
Disclosed is a novel and excellent therapeutic or prophylactic agent for nociceptive pain, neuropathic pain, cancer pain, headache, bladder dysfunction or the like, which relies on a preventive activity on capsaicin receptor VR1 activation. It is found that a benzamide derivative having a benzene ring fused with a monocyclic ring on a nitrogen atom in the amide group and an amino group substituted by a lower alkylamino or cyclic group at a position adjacent to the amide group has a potent preventive activity on VR1 activation and an excellent pharmacological activity relying on the preventive activity, and therefore the derivative can be used as a good therapeutic or prophylactic agent for a VR1-related disease, such as nociceptive pain, neuropathic pain, cancer pain, headache and bladder dysfunction.
Description
technical field The present invention relates to a novel 2-aminobenzamide derivative or a salt thereof useful as a drug, especially as an activation inhibitor of capsaicin receptor VR1 (Vanilloid Receptor 1), and a drug thereof. Background technique VR1 is a receptor present on primary afferent sensory nerves (mainly C fibers), involved in pain in various diseases. This receptor is activated by capsaicin, which is the main component of tang xinzi, causing pain. It is known that in VR1-deficient mice, not only the pain response induced by capsaicin disappears, but also hyperalgesia during inflammation is attenuated [Nature 405: 183-187 (2000)]. It is known that capsaicin causes pain by activating VR1 as described above, but due to continuous activation desensitizes the afferent nerve, and inhibits subsequent activation, it shows analgesic effect [Pharmacol.Rev.51: 159-211 (1999 ); Drugs Aging 18:561-573 (2001)]. In fact, capsaicin cream is used in the treatment of neuroge...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/167A61K31/404A61K31/428A61K31/4439A61K31/454A61K31/4704A61K31/4709A61K31/496A61K31/5377A61K31/538A61K31/5415A61P13/10A61P25/02A61P29/00A61P43/00C07D207/09C07D207/14C07D209/34C07D211/06C07D211/26C07D211/58C07D213/80C07D215/38C07D265/30C07D265/36C07D277/62C07D277/64C07D279/16C07D295/12C07D295/14C07D307/14C07D307/22C07D309/14C07D319/12C07D401/12C07D405/12C07D413/12C07D417/12
CPCC07C37/18C07D295/185C07D211/58C07D403/12C07D265/36C07C237/40C07D215/38C07D401/14C07D307/22C07D307/14C07D413/04C07D321/10C07D209/96C07D207/09A61K31/167C07C2101/14C07D215/227C07D453/02C07D319/18C07D209/48C07D319/12C07C2102/10C07D217/02C07D213/38C07D279/16C07D213/74C07D405/12C07D207/14C07D413/12C07D277/64C07D295/135C07D401/12C07D309/14C07D209/34C07D405/14C07D417/12C07D213/80C07D295/13C07C2601/14C07C2602/10A61P13/10A61P25/02A61P25/04A61P25/06A61P29/00A61P43/00C07C39/27A61K31/404A61K31/428
Inventor 仓持孝博平林亮治小金丸阳平宗像亮介米泽公一木曾哲男
Owner ASTELLAS PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com