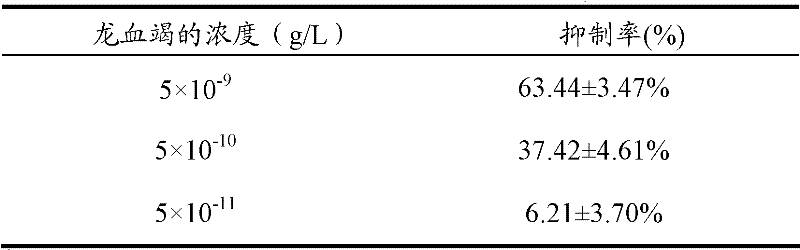Application of dragon's blood and flavonoid chemical component thereof in serving as antagonist of capsaicin receptor TRPV1 (transient receptor potential vanilloid 1)
A capsaicin receptor and chemical composition technology, applied in the fields of pharmacology and biology, can solve the problems that the modulation effect has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: dragon's blood preparation
[0046] Prepare extracellular fluid first: Take 8.4824g of NaCl, 2.3832g of HEPES, 1.9816g of D-Glucose, 0.3728g of KCl, 0.222g of CaCl, 20.2032g of MgCl, add 800ml of purified water to fully dissolve, adjust the pH value of the solution to 7.4 with NaOH, and then add Dilute purified water to 1L and use it after filtering through a microporous membrane with a diameter of 0.45 μm.
[0047] Dissolve 5 mg of dragon's blood powder in 1 mL of dimethyl sulfoxide solution to make 5‰ dragon's blood dry liquid, and take 1 μL of dragon's blood dry liquid to dissolve in 1 L of extracellular fluid to make 1 L with a concentration of 5×10 -9 g / L dragon's blood preparation.
Embodiment 2
[0048] Embodiment 2: Stenophyllin A preparation
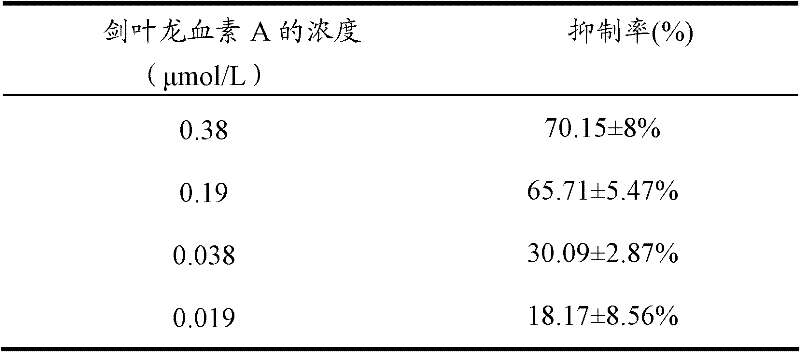
[0049] According to the method of Example 1, the extracellular fluid was prepared, and 0.0286 g of phoenixin A powder was dissolved in 10 mL of dimethyl sulfoxide solution to make a 10 mmol / L dry solution of cyphophyllin A, and 3.8 μL of phophyllin A was taken. The dry solution of astragalusin A was dissolved in 0.1L of extracellular fluid to make 0.1L of cyphophyllin A preparation with a concentration of 0.38 μmol / L
Embodiment 3
[0050] Example 3: Mixed preparations of cypholongerin A and phopholyptin B
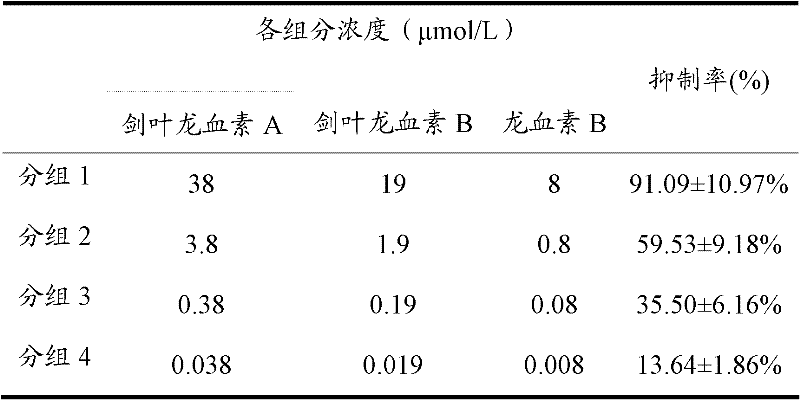
[0051]According to the method of Example 1, the extracellular fluid was prepared, and 0.0286 g of phoenixin A powder was dissolved in 10 mL of dimethyl sulfoxide solution to make a 10 mmol / L dry liquid of cyphophyllin A. Dissolve 0.0286g of glucoside powder in 10mL dimethyl sulfoxide solution to make 10mmol / L dry solution of photolongin B, and take 38μL dry solution of photolongin A and 19μL dry solution of phodolongin B Dissolved in 0.1L of extracellular fluid to make 0.1L of eolongin A and phophoramin B mixed preparation, which contains phopholongin A at a concentration of 3.8 μmol / L and phopholongin B at a concentration of 1.9 μmol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com