New medicinal use of nintedanib, and derivative and medicinal salt thereof
A technology of nintedanib and derivatives, which is applied in the field of pharmaceutical applications to achieve strong pharmacological effects, good safety, and the effects of improving treatment and prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
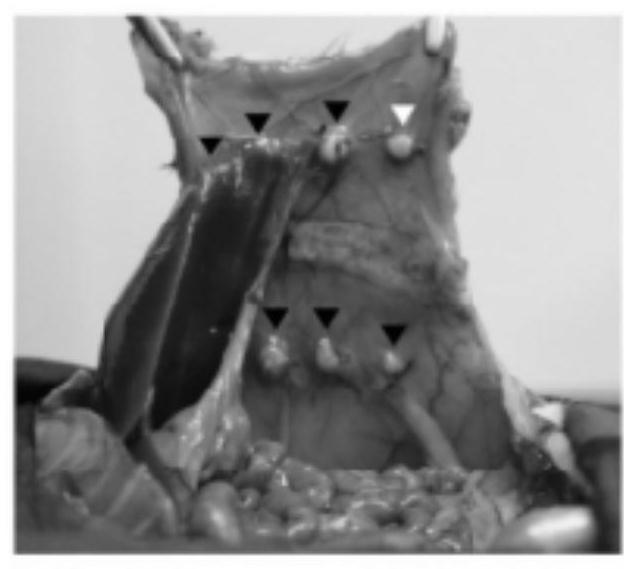
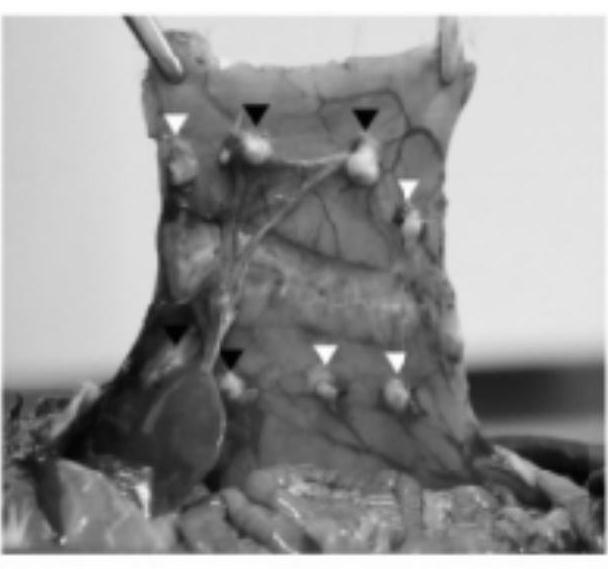
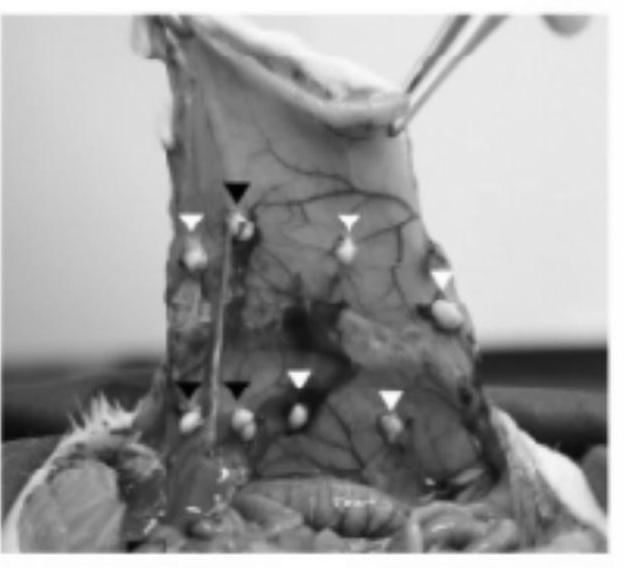
[0028] Example 1: Animal experiments on the preventive and therapeutic effects of nintedanib on a rat model of peritoneal adhesions
[0029] 1. Experimental materials
[0030] 1.1 Experimental animals: 18 SD rats (SPF grade, female, 220g-260g), source and animal qualification certificate: Beijing Huafukang Biotechnology Co., Ltd.; SCXK (Beijing) 2019-0008, NO.1103221911015030.
[0031] 1.2 Reagent: Chloral, hydrate: C804539, Shanghai Macklin Biochemical Company; 10% chloral hydrate: use material 2) and pure water to prepare 10% chloral hydrate solution; iodophor disinfectant: batch number 200528W, Changjiang, Beijing Mai Medicine Technology Co., Ltd.; Nintedanib (purity 99.97%): Item No.: HY-50904, MedChemExpress (MCE) Company; Medical 0.9% Sodium Chloride Solution (Normal Saline): Batch No.: C202005121, China Resources Double-Crane Pharmaceuticals limited company.
[0032] 1.3 Equipment: surgical instruments: tissue scissors (J22010, Shanghai Medical Instrument (Group) Co.,...
Embodiment 2
[0050] Example 2: Pathological evaluation experiment of nintedanib on local inflammatory response and fibrosis response of peritoneal adhesion tissue in rats
[0051] 1. Experimental materials
[0052] 1.1 Source of pathological tissue: ischemic nodular tissue and peritoneal adhesion tissue of experimental rat abdominal wall in rat peritoneal adhesion experiment
[0053] 1.2 Reagent: 10% Neutral Formalin Fixative: Item No.: Top0372, Beijing Biotuoda Technology Co., Ltd.; Medical 0.9% Sodium Chloride Solution (Normal Saline): Batch No.: C202005121, China Resources Double Crane Pharmaceutical Co., Ltd. ; Anhydrous ethanol solution (for molecular biology): Product No.: E111991, Shanghai Aladdin Biochemical Technology Co., Ltd.; Xylene solution: Product No.: 1330-20-7, Sinopharm Chemical Reagent Co., Ltd.; hematoxylin-eosin Staining kit (H&E Staining kit): product number: ab245880, Abcam, USA.
[0054] 1.3 Equipment: Leica manual rotary pathological slicer: Model: RM2235, Leica ...
Embodiment 3
[0067] Embodiment three: the preparation of nintedanib tablet
[0068] Take 100g of nintedanib, mix it with conventional adjuvants, and prepare 1000 nintedanib tablets according to conventional methods to obtain nintedanib tablets with a specification of 100 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com