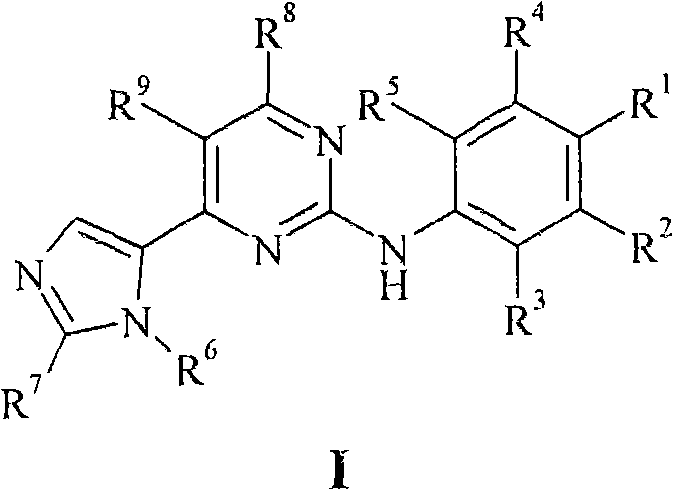
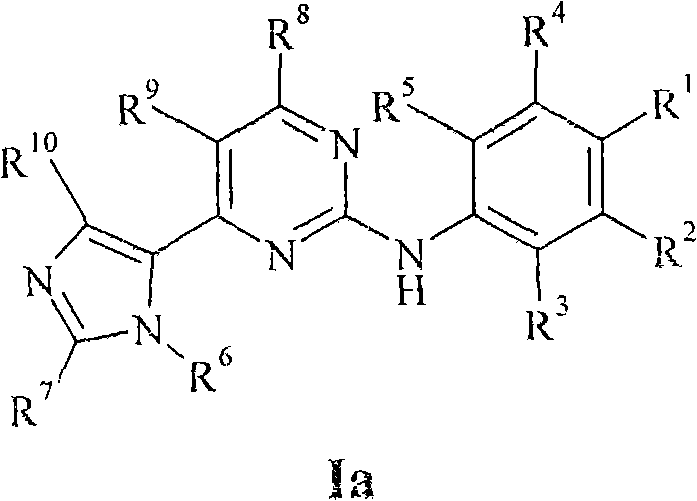
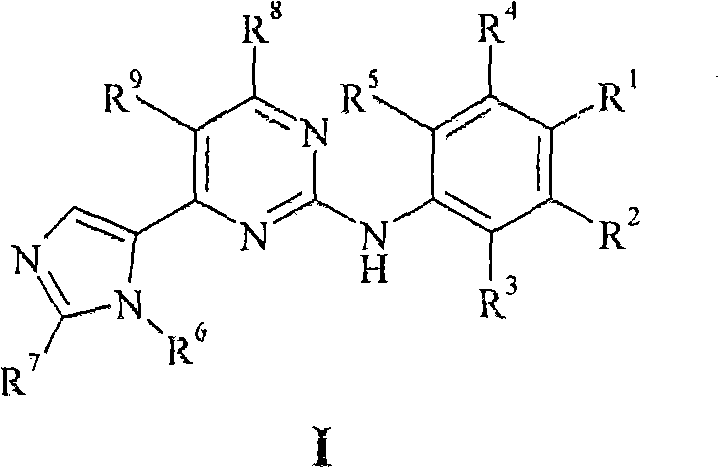
New pyrimidine derivatives and their use in therapy as well as the use of pyrimidine derivatives in the manufacture of a medicament for prevention and/or treatment of alzheimer's disease
A technology of compounds and medicinal salts, which can be applied to medical preparations containing active ingredients, drug combinations, metabolic diseases, etc., and can solve problems such as lithium poisoning and narrow therapeutic windows
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0596] 4-(1,2-Dimethyl-1H-imidazol-5-yl)-5-fluoro-N-[3-methoxy-5-(trifluoromethyl)phenyl]pyrimidin-2-amine
[0597] Embodiment 1 (a) 1,2-dimethyl-5-(trimethyltin base)-1H-imidazole
[0598]
[0599]1,2-Dimethylimidazole (0.960 g, 10.0 mmol) was diluted in anhydrous THF (50 mL) under argon, and the solution was cooled to -78°C. tert-Butyllithium (1.7M in pentane, 6.47 mL, 11.0 mmol) was added dropwise over 5 minutes. The reaction mixture was stirred at -78 °C for 1 h, then treated with a solution of trimethyltin chloride (2.2 g, 11.0 mmol) in anhydrous THF (10 mL). The mixture was stirred at -78°C to room temperature for 60 hours. The solvent was then evaporated in vacuo to give the title compound (1.29 g, 50%). The crude product was used in the next step without further purification.
[0600] 1 H NMR (CDCl 3 )δppm 6.87 (s, 1H), 3.56 (s, 3H), 2.41 (s, 3H), 0.45-0.18 (m, 9H); MS (CI) m / z 261 ( 120 Sn)(M+1).
[0601] Embodiment 1 (b) 2-chloro-4-(1,2-dimethyl-1H-imidazo...
Embodiment 2
[0610] N-(3,5-dichlorophenyl)-4-(1,2-dimethyl-1H-imidazol-5-yl)-5-fluoropyrimidin-2-amine
[0611]
[0612] The title compound was followed in general procedure C using 2-chloro-4-(1,2-dimethyl-1H-imidazol-5-yl)-5-fluoropyrimidine (from Example 1(b)) (50 mg, 0.221 mmol ) and 3,5-dichloroaniline (39mg, 0.243mmol) to give the title compound (15mg, 19%).
[0613] 1 H NMR (DMSO-d 6 )δppm 10.12(s, 1H), 8.74(d, J=2.8Hz, 1H), 7.96(d, J=2.8Hz, 1H), 7.79(d, J=2.0Hz, 1H), 7.72-7.55(m , 1H), 7.16 (t, J = 1.8 Hz, 1H), 4.00 (s, 3H), 2.57 (s, 3H); MS (ESI) m / z 352 (M+1).
Embodiment 3
[0615] (4-{[4-(1,2-Dimethyl-1H-imidazol-5-yl)-5-fluoropyrimidin-2-yl]amino}phenyl)(phenyl)methanone
[0616]
[0617] The title compound was followed in general procedure C using 2-chloro-4-(1,2-dimethyl-1H-imidazol-5-yl)-5-fluoropyrimidine (from Example 1(b)) (80 mg, 0.354 mmol ) and (4-aminophenyl)(phenyl)methanone (84mg, 0.424mmol), Pd(OAc) 2 (4.7mg, 0.021mmol) and Pd(t-Bu 3 P) 2 (10.7 mg, 0.021 mmol) to give the title compound (56 mg, 41%).
[0618] 1 H NMR (CDCl 3 )δppm 8.32 (s, 1H), 8.00-7.63 (m, 7H), 7.60-7.33 (m, 4H), 3.96 (s, 3H), 2.51 (s, 3H); MS (ESI) m / z 388 ( M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com