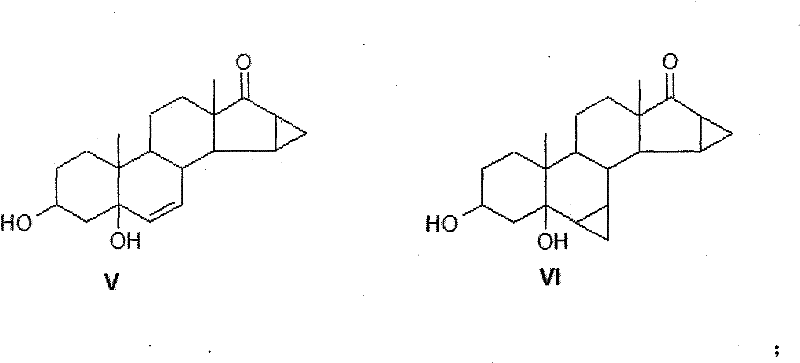
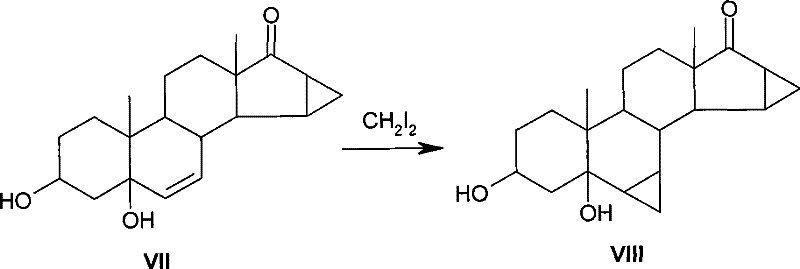
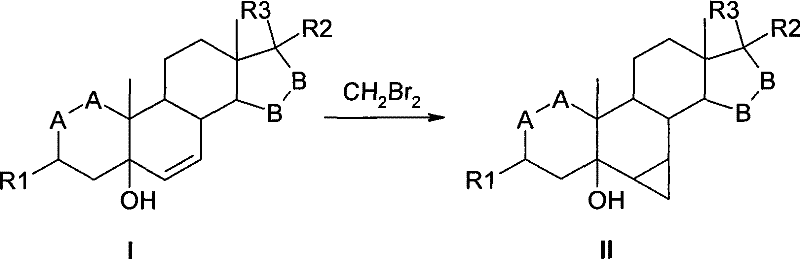
Method for synthesis of 6,7-methylene sterides
A technology of methylene steroids and steroidal compounds, applied in the direction of steroids, organic chemistry, etc., can solve the problems of expensive diiodomethane, limited use, large amount of diiodomethane, etc., and achieve large-scale industrial production value, The effect of low production cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Under the protection of inert gas, dissolve 5g of 3β, 5-dihydroxy-6,7-double bond-15β, 16β-methylene-5β-androst-17-one in 100ml of ethylene glycol dimethyl ether, add 25g Zn-Cu reagent, add 0.25g of iodine as an initiator, stir and heat to 40°C, add 10ml of dibromomethane dropwise, a large number of bubbles will be generated at the beginning, after the reaction is slightly relaxed, continue to add dropwise, the internal temperature rises to about 70°C, 20 The drip is over in minutes.
[0034] After dropping, keep the reaction at 70° C. for 1 hour.
[0035] Follow the reaction by TLC (developer: cyclohexane: ethyl acetate = 1:2), if there are still raw material spots, continue to add 2.5 ml of dibromomethane dropwise. After the dropwise reaction was completed, the reaction was carried out at 70° C. for 1 hour. After the reaction was followed by TLC, it was cooled to room temperature. Diatomaceous earth was used to filter, and the filter cake was washed with a small am...
Embodiment 2
[0037] Under the protection of inert gas, dissolve 5g of 3β, 5-dihydroxy-6,7-double bond-15β, 16β-methylene-5β-androst-17-one in 100ml of ethylene glycol dimethyl ether, add 25g Zn-Cu reagent, add 0.25g of iodine as an initiator, stir and heat to 40°C, add 12ml of dibromomethane dropwise, a large number of bubbles will be generated at the beginning, after the reaction is slightly moderate, continue to add dropwise, the internal temperature rises to about 70°C, 25 The drip is over in minutes.
[0038] After dropping, keep the reaction at 70° C. for 1 hour. Follow the reaction by TLC (developer: cyclohexane: ethyl acetate = 1:2), if there are still raw material spots, continue to add 2.5 ml of dibromomethane dropwise. After the dropwise reaction was completed, the reaction was carried out at 70° C. for 1 hour. Post-processing is the same as above. The product was dried to obtain 3.5 g, the yield was 67%, and the purity by HPLC was 88%.
Embodiment 3
[0040] Under the protection of inert gas, dissolve 5g of 3β, 5-dihydroxy-6,7-double bond-15β, 16β-methylene-5β-androst-17-one in 100ml of ethylene glycol dimethyl ether, add 25g Zn-Cu reagent, add 0.25g of iodine as an initiator, stir and heat to 40°C, add 8ml of dibromomethane dropwise, a large number of bubbles will be generated at the beginning, after the reaction is slightly relaxed, continue to add dropwise, the internal temperature rises to about 70°C, 15 The drip is over in minutes.
[0041] After dropping, keep the reaction at 70° C. for 1 hour. Follow the reaction by TLC (developer: cyclohexane: ethyl acetate = 1:2), if there are still raw material spots, continue to add 2 ml of dibromomethane dropwise. After the dropwise reaction was completed, the reaction was carried out at 70° C. for 1 hour. Post-processing is the same as above. After drying, 3.2 g of the product was obtained, with a yield of 61%, and a purity of 85% as detected by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com