Stable tartaric acid ifenprodil injection and method of preparing the same
A technology of ifendil tartrate and injection, which is applied in the directions of inactive medical preparations, pharmaceutical formulations, inorganic inactive ingredients, etc., to achieve the effects of reducing the risk of adverse reactions and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Ifenprodil tartrate 5g,
[0027] tartaric acid or sodium hydroxide to adjust pH6.7~7.2,
[0028] Water for injection 2000ml
[0029] a. According to the above formulation prescription, weigh 30g of sodium sulfate, add 2000mL of water for injection at 40°C to 80°C to dissolve.
[0030] b. Use tartaric acid or sodium hydroxide to adjust the pH of the solution to 6.7-7.2, add 5 g of ifenprodil tartrate, and dissolve.
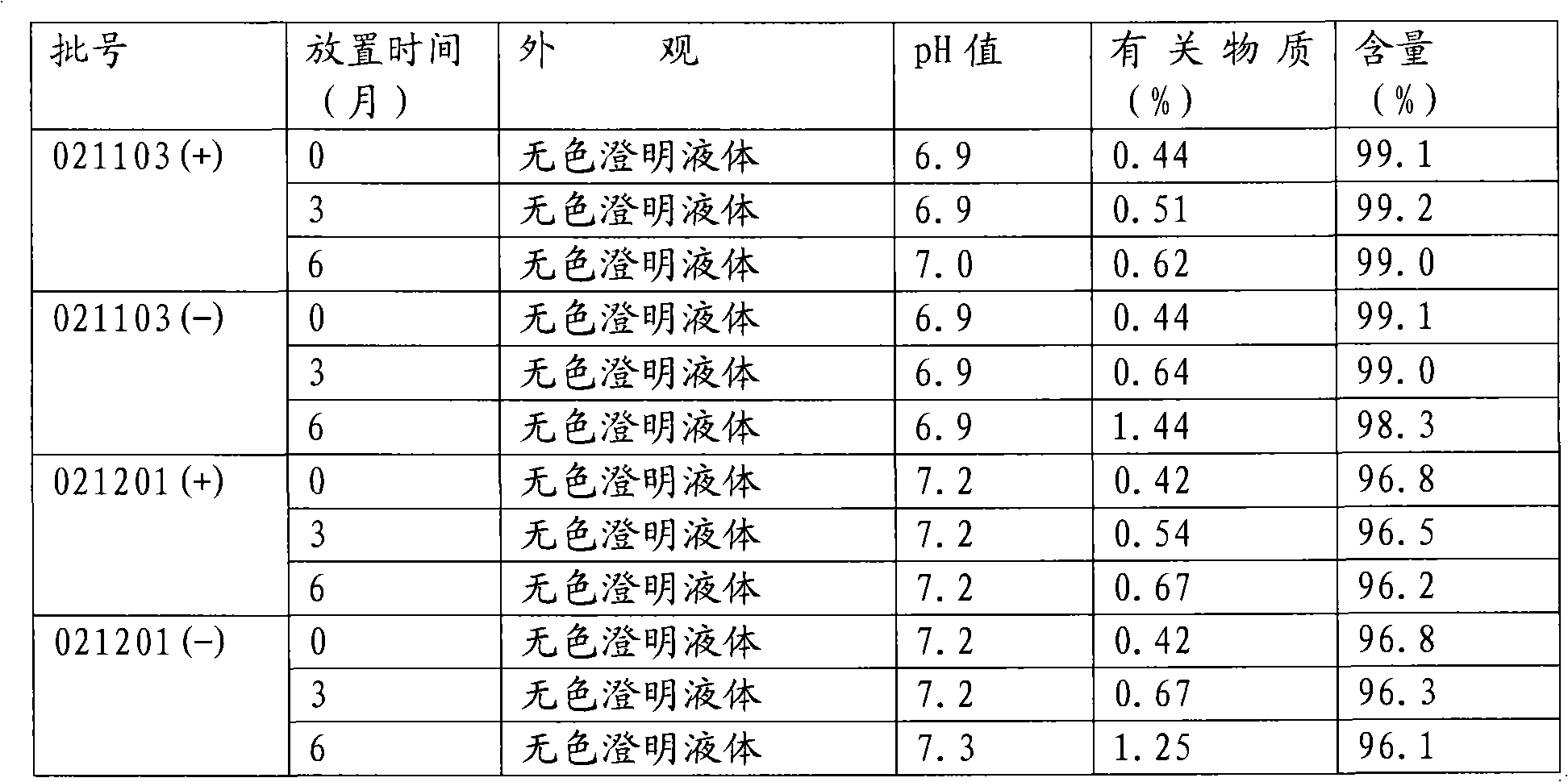
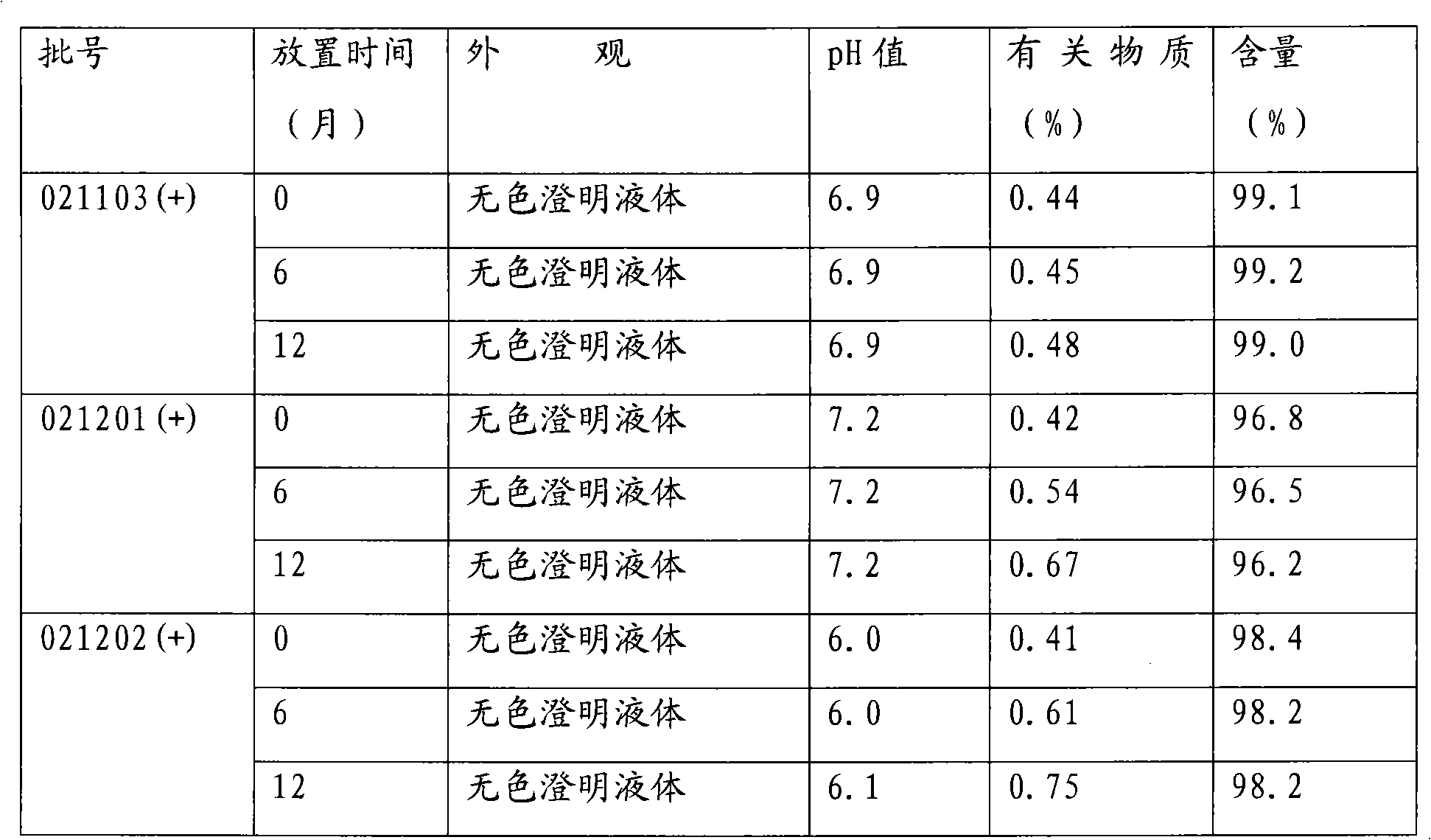
[0031] c. Filter with a 0.8um microporous membrane, test the pH, clarity and content of the filtrate, and pack it into clean ampoules after passing the test, with a volume of not less than 2.15ml.
[0032] d. Seal the semi-finished product with nitrogen gas.
[0033] Sterilize in a 100°C water bath for 40 minutes and check for leaks.
Embodiment 2
[0035] Ifenprodil tartrate 20g,
[0037] Hydrochloric acid or sodium hydroxide to adjust pH6.7~7.2,
[0038] Water for injection 2000ml
[0039] a. According to the above formulation prescription, weigh 22g of sodium sulfate, add 2000mL of water for injection at 40°C to 80°C to dissolve.
[0040] b. Use hydrochloric acid or sodium hydroxide to adjust the pH of the solution to 6.7-7.2, add 20 g of ifenprodil tartrate, and dissolve.
[0041] c. Filtrate with a 0.8um microporous membrane, check the pH, clarity and content of the filtrate, and pack it into clean ampoules after passing the test. The filling volume should not be less than 2.15ml.
[0042] d. The semi-finished product is obtained by fusing and sealing with argon gas.
[0043] Sterilize in a 100°C water bath for 40 minutes and check for leaks.
Embodiment 3
[0045] Ifenprodil tartrate 20g,
[0046] Sodium sulfate 139g,
[0047] tartaric acid or sodium hydroxide to adjust pH6.7~7.2,
[0048] Water for injection 10000ml
[0049] a. According to the above formulation prescription, weigh 139g of sodium sulfate, add 10000mL of water for injection at 40°C to 80°C to dissolve.
[0050] b. Use tartaric acid or sodium hydroxide to adjust the pH of the solution to 6.7-7.2, add 20 g of ifenprodil tartrate, and dissolve.
[0051] c. Filter with a 0.8um microporous membrane, test the pH, clarity and content of the filtrate, and pack it into clean ampoules after passing the test, with a volume of not less than 2.15ml.
[0052] d. Seal the semi-finished product with nitrogen gas.
[0053] Sterilize in a 100°C water bath for 40 minutes and check for leaks.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com