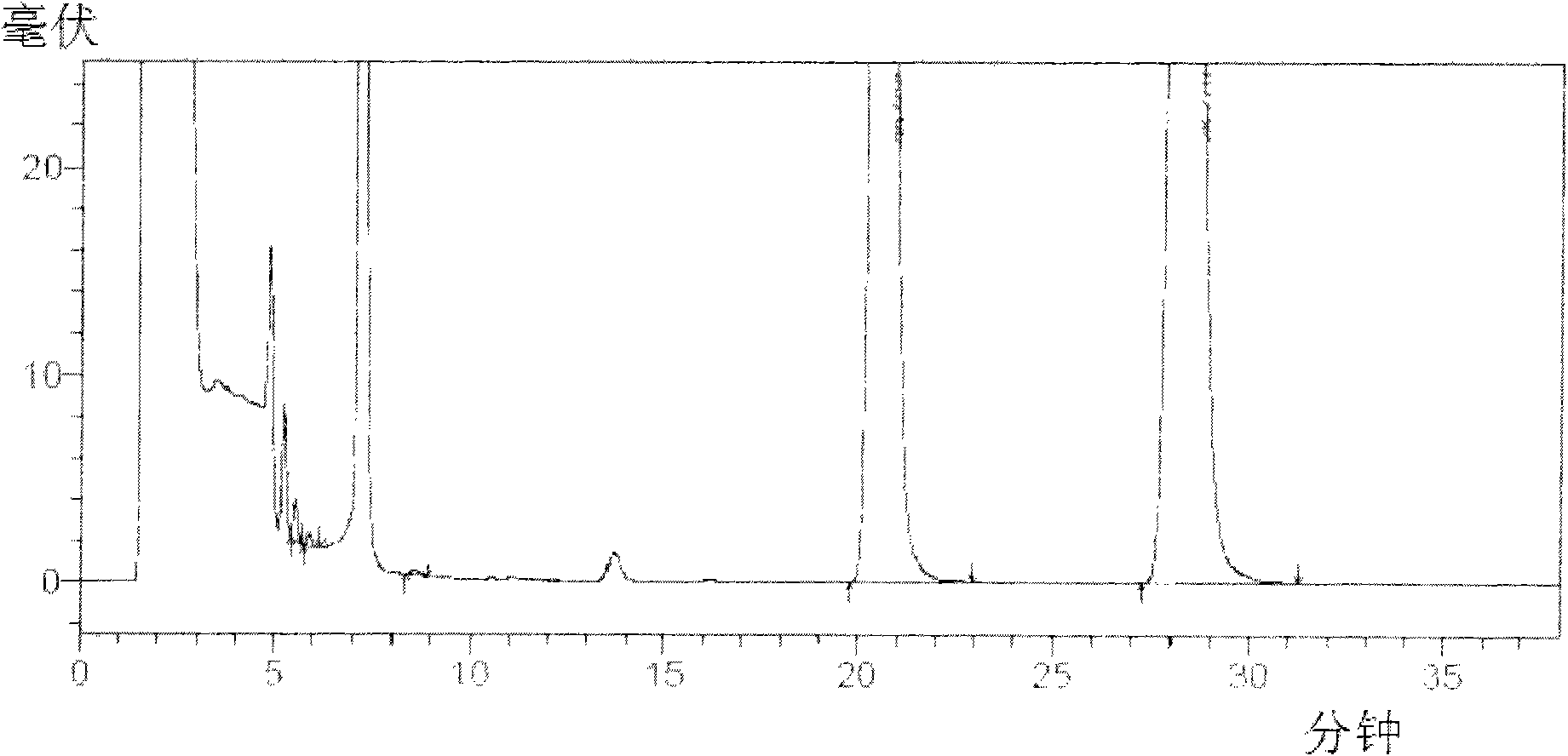
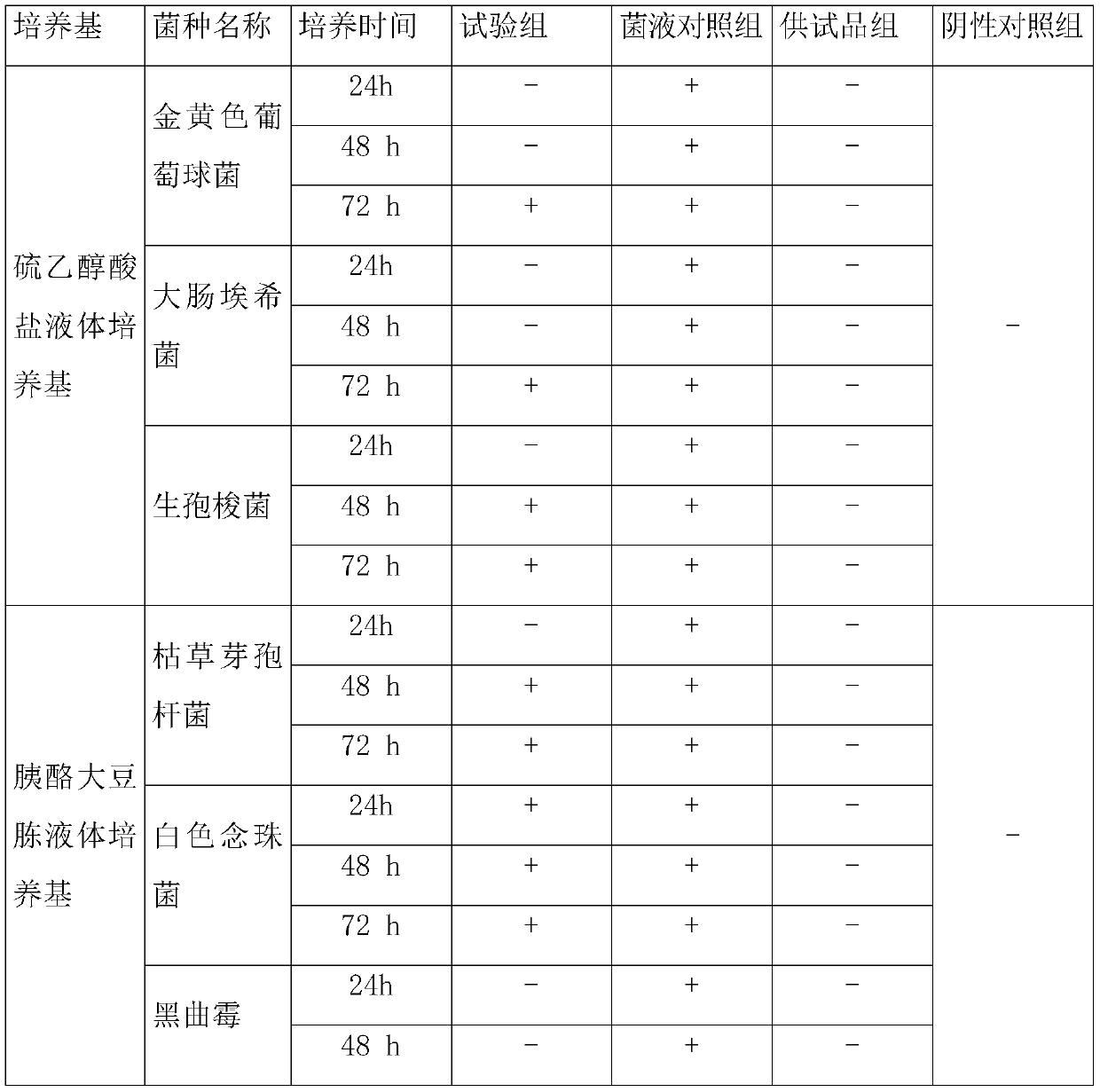
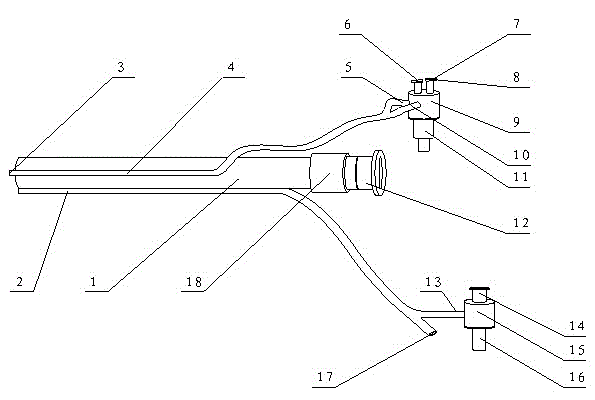
Patents
Literature
69 results about "Sterility test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
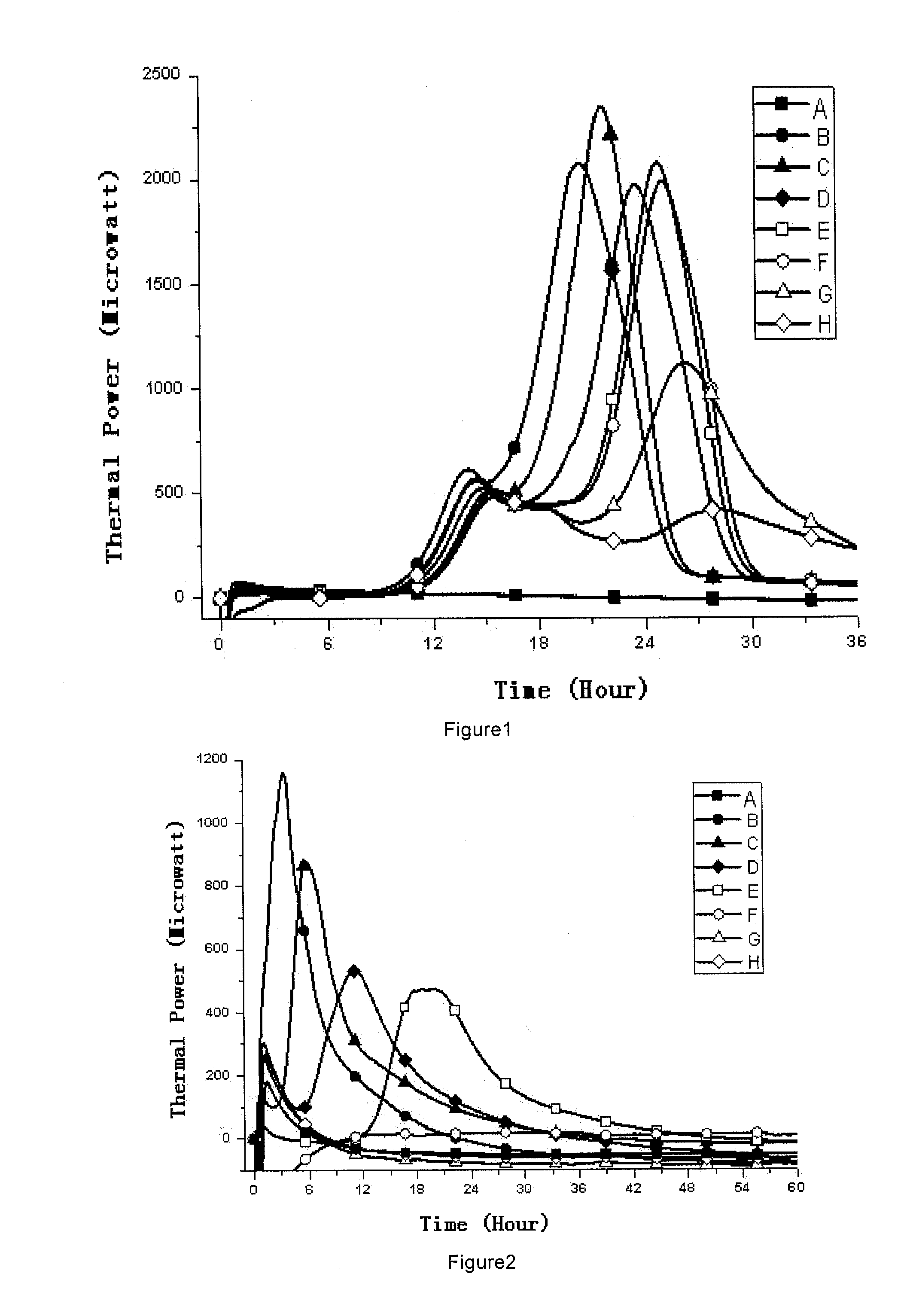
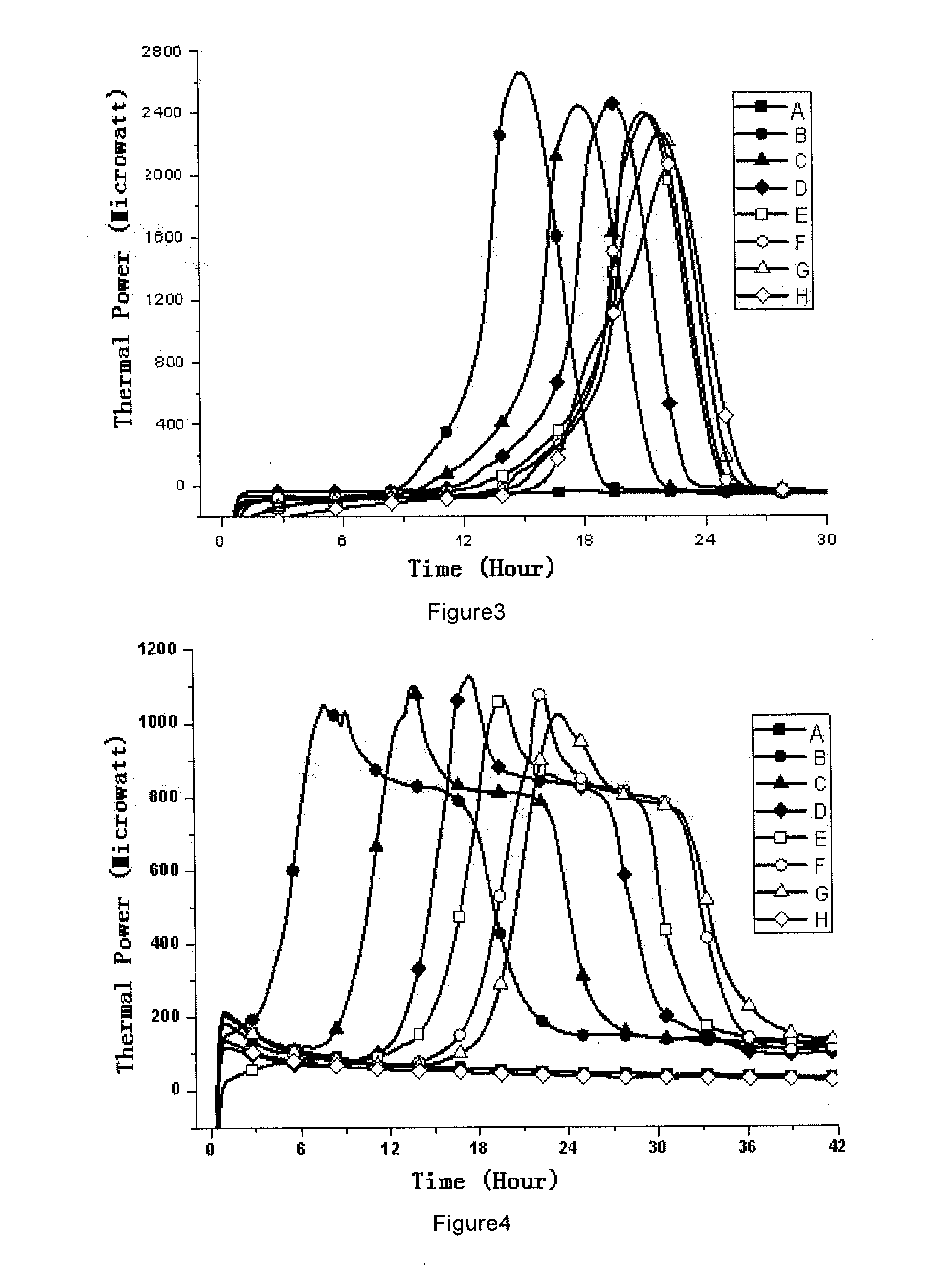
Sterility test is the main test for the sterile products and it also takes a lot of time to complete. Repeat of the test can cause problem for the manufacturing unit due to long time taken in analysis.
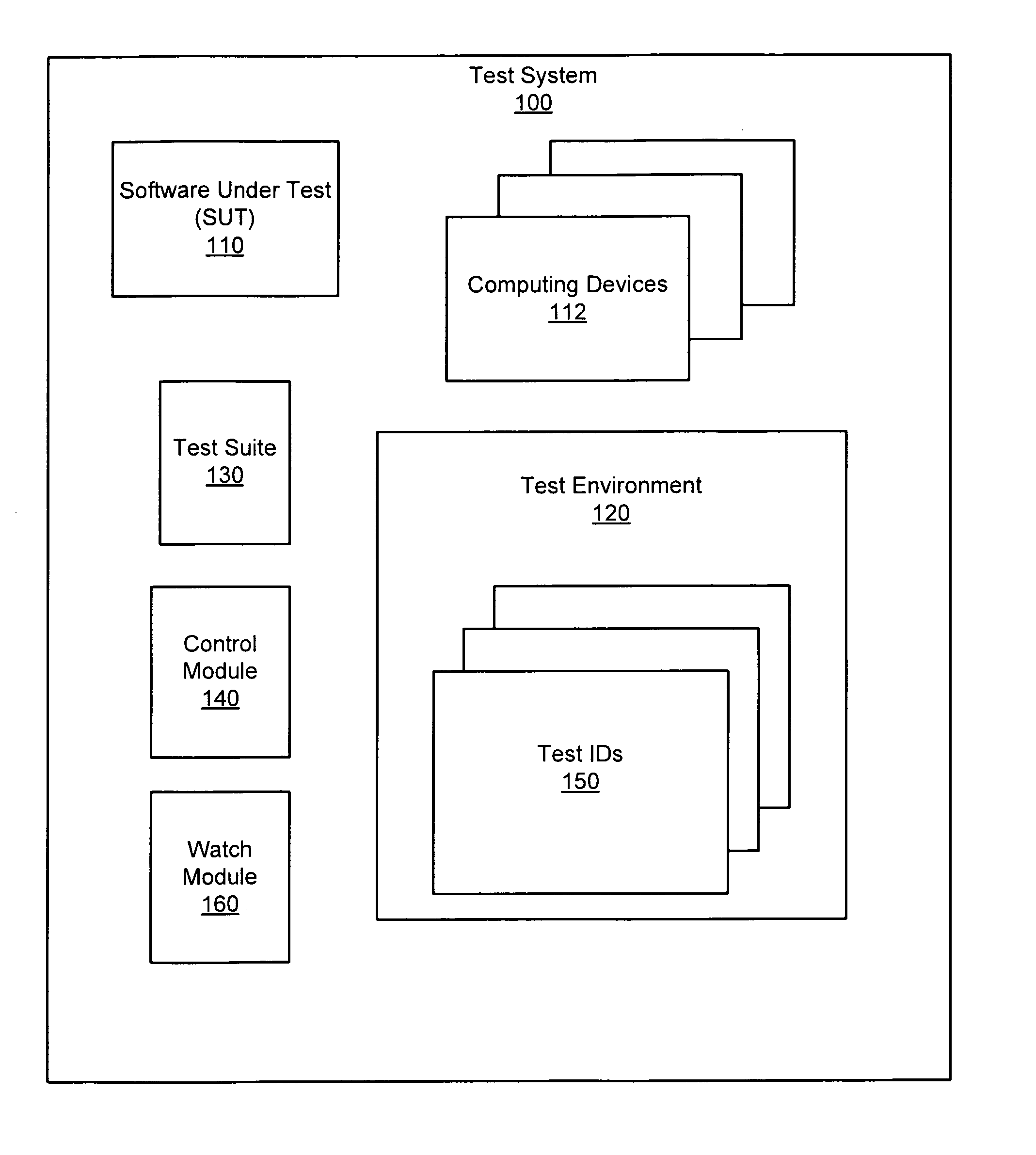
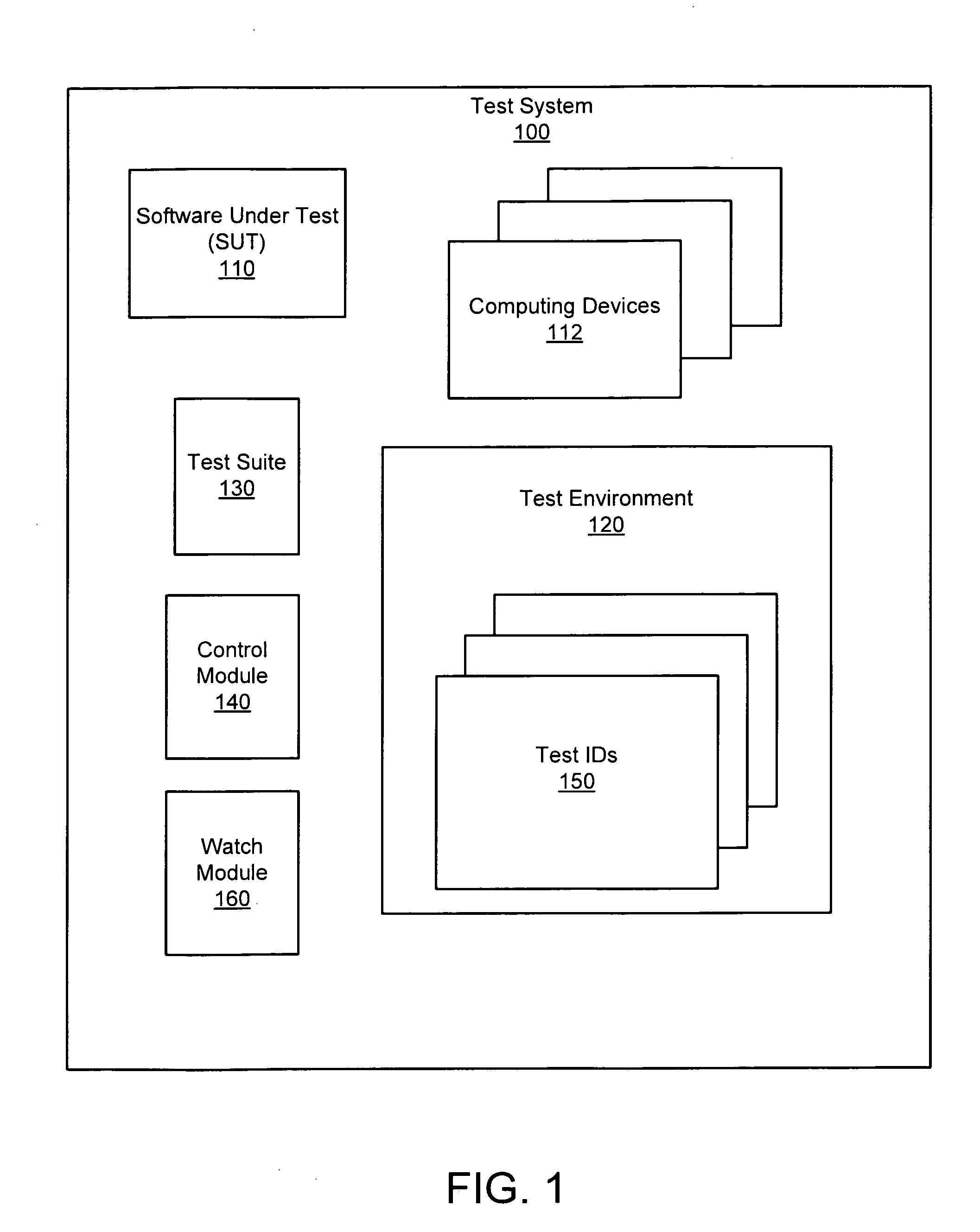
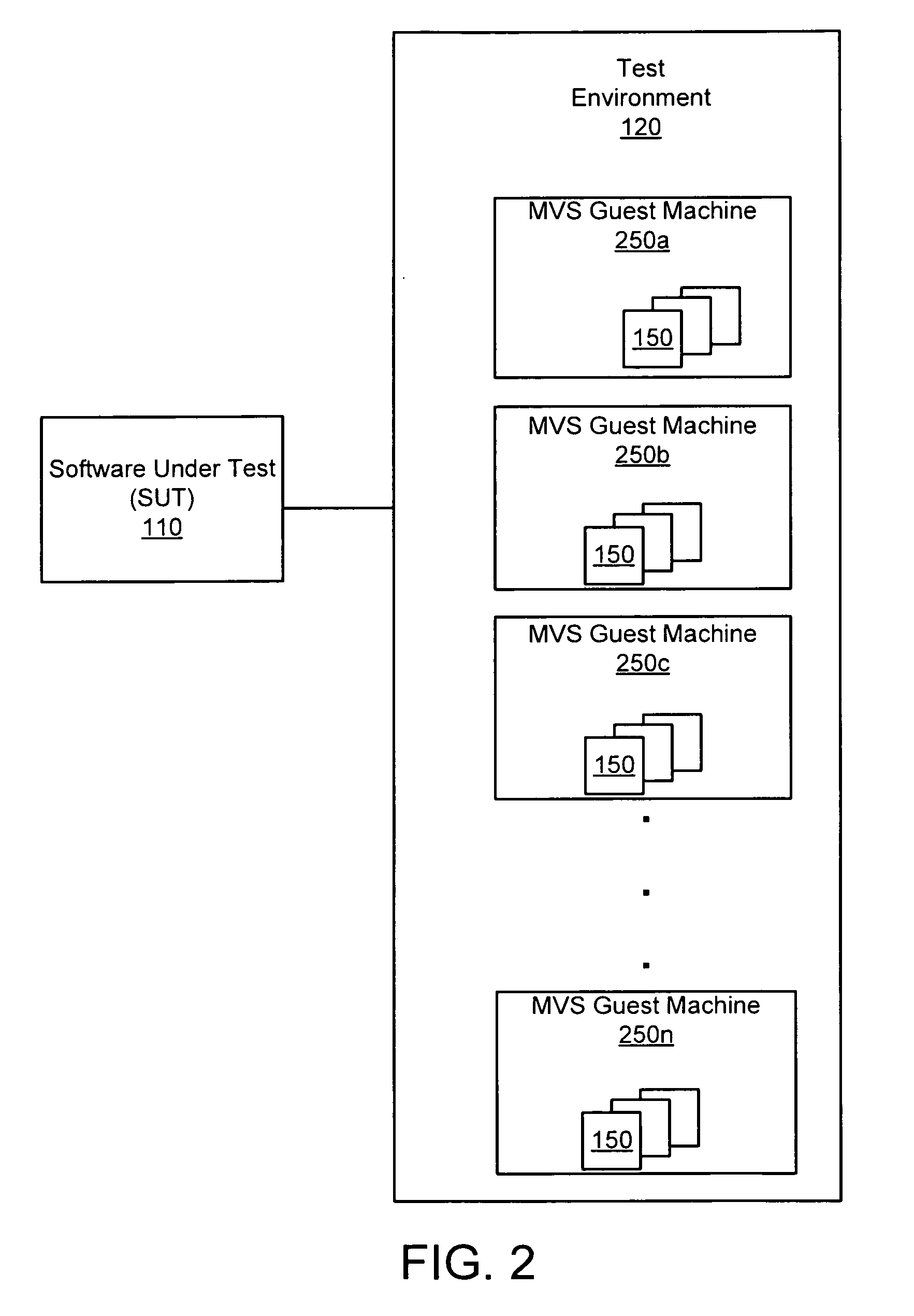
Apparatus, system, and method for persistent testing with progressive environment sterilzation
An apparatus, system, and method are disclosed for automatically testing a plurality of software test cases. The testing executes a quick test of the test cases which executes each test case in a test environment that is initialized just prior to the first test case and after subsequent test case failures. The testing further executes an adjusted test of the failing test cases in which delay parameters associated with the failing test cases are increased in accordance with a system load recorded during the quick test. Finally, the testing executes a sterilized test of the remaining failing test cases in a test environment that is initialized prior to each test case execution.
Owner:IBM CORP
Sterility test method and totally enclosed bacterial ampoule incubator used by it
InactiveUS20130109052A1Increase labor costLow costBioreactor/fermenter combinationsBiological substance pretreatmentsDrainage tubesCulture mediums
A sterility test method includes: selecting strain and culture medium, preparing bacterial cultures, transcribing fingerprint characteristics in thermograms as indices to verify the characteristics, drawing the thermodynamic parameters of the thermogram, determining the positive judgment index and performing sterility test for the samples. A fully-enclosed bacteria collecting ampoule incubator includes bacteria collecting ampoule system, sample and liquid feeding system and peristalsis liquid discharge system. The sample and liquid feeding system is connected with the bacteria collecting ampoule system by the liquid intake tube; and the bacteria collecting ampoule system is connected with the peristalsis liquid discharge system by the liquid drainage tube. The invention is characterized by short inspection time, high sensitivity, high automation and accurate test results on microbial contamination. It can also provide the overall process curve on the growth conditions. Such curve is provided with relatively favorable fingerprint, which enables qualitative analysis on the microbial contamination conditions.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
Separation screening method for antibiotic antituberculotic plant endophyte
InactiveCN101225430AEasy to operateMicrobiological testing/measurementMicroorganism separationMicroorganismPlant tissue
The invention discloses a separation and selection method of antibiotic antitubercular endophyte, which comprises the steps as follows, the preparation of antibiotic antitubercular plant tissue, the surface sterilization and the sterility test, the separation and the purification of the endophyte, the ferment of endogenetic fungi, the ferment of endogenetic bacterium, the process of the zymotic fluid, the process of the thalli, the selection of the effective antibiotic bacterial strains, the selection of the effective antitubercular bacterial strains and the preservation of the strains. The separation and selection method of antibiotic antitubercular endophyte ensures the realization of obtaining the effective antibiotic antitubercular matter from microorganisms, thanks to separation and selection method, a batch of effective bacterial strains are obtained.
Owner:JIANGSU UNIV OF SCI & TECH
Method for storing endometrial stem cells
InactiveCN102154202AIncrease the number ofHigh purityDead animal preservationSkeletal/connective tissue cellsVolumetric Mass DensityCell separation
The invention discloses a method for storing endometrial stem cells, which comprises the following steps: 1) preparing a collection tool set and preparing a culture medium; 2) collecting menses; 3) performing a sterility test; and 4) subjecting endometrial stem cell to separation culture, namely, filtering mixed liquid, which is obtained by the step 2), in a collection tube 1, collecting single karyoplasts by using a density gradient centrifugation method, inoculating the obtained single karyoplasts in a Chang complete culture medium at an inoculation density of single karyoplasts of 1*10<5> to 1*10<6> / ml, and culturing in a culture tank which is at 37 DEG C and saturated humidity and contains CO2 at a volume percentage concentration of 5 percent; 5) amplifying and purifying cells; and 6) storing cells by freezing. When the method of the invention is used, a large number of high-purity target cells, namely endometrial stem cells, can be obtained.
Owner:HANGZHOU S EVANS BIOSCI LTD
Gynecological medical gel dressing and preparation method thereof
InactiveCN103301502AGood biocompatibilityImprove biological activityAbsorbent padsBandagesSodium bicarbonateDisease
The invention discloses a gynecological medical gel dressing and a preparation method thereof, and aims at providing a gynecological medical gel dressing being remarkable in effect of treating various gynecologic inflammations and capable of quickly repairing disseminated congestion, mucosal injury and superficial ulcer caused by vagina inflammatory diseases and cervical erosion. According to the technical points, the dressing comprises chitosan at a concentration of 1.25 to 2.0% and other auxiliary materials; and the preparation method comprises the following steps of: weighing chitosan and dissolving by 0.5% of lactic acid solution to obtain chitosan liquid; slowly adding carbomer accounting for 1% of the total mass and glycerinum accounting for 10% of the total mass to the chitosan liquid; uniformly stirring; adding chlorhexidine acetate accounting for 0.1% of the total mass; uniformly mixing; adjusting pH (Potential of Hydrogen) to 4.5 to 7.5 through a sodium bicarbonate solution; filtering; removing bubble through vacuum equipment to obtain a semi-finished product; detecting biological indexes and physicochemical indexes the next day; performing split charging through a hose syringe; carrying out sterility test, thus obtaining the finished product after confirmation. The gynecological medical gel dressing and the preparation method thereof belong to the technical field of pharmaceutical preparation.
Owner:王珍
Stable tartaric acid ifenprodil injection and method of preparing the same
InactiveCN101347429AEasy to solveLow costNervous disorderInorganic non-active ingredientsOxygenInjection solution
The invention discloses a stable ifenprodil tartrate injection which contains ifenprodil tartrate, an osmotic pressure regulator, a pH regulator and water for injection. The invention also discloses a preparation method of the stable injection. The stable ifenprodil tartrate injection is obtained by controlling the range of the pH value of the solution and insulating oxygen, thus avoiding use of an antioxidant and possible adverse reactions thereof. Sterility tests, accelerated tests and long duration tests prove the stable ifenprodil tartrate injection.
Owner:重庆人本药物研发有限责任公司 +1
Foot-and-mouth disease virus antigen preservation technology
A technique for preserving the antigen of foot-and-mouth disease virus includes such steps as preparing its protecting agent from hydrolytic actoprotein liquid, glycerine, peptone and cane sugar through proportional mixing and high-pressure sterilizing, sterility test, adding it to the foot-and-mouth disease virus liquid, and preserving at -20- -70 deg.C or in liquefied nitrogen (-196 deg.C).
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Aeromonas hydrophila inactivated vaccine and preparation thereof
InactiveCN101642567AOutbreak Prevention and ControlEpidemic prevention and controlAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsAeromonas hydrophila
The invention relates to a fish vaccine and a preparation method thereof, in particular to an aeromonas hydrophila inactivated vaccine and the preparation thereof. The aeromonas hydrophila inactivatedvaccine is prepared by the following concrete steps: separating out a pathogen from a sick fish, determining the pathogen as an aeromonas hydrophila through morphology, virulence factors, biochemicalreaction and gene sequencing; rejuvenating the strain, selecting an optimum condition for cultivation; then selecting the optimum condition for inactivation to prepare the aeromonas hydrophila inactivated vaccine, and conforming the aeromonas hydrophila inactivated vaccine to be certificated through sterility test and security test. The inactivated vaccine is prepared from a pathogenic bacterialstrain separated from a sick fish body, has a simple operating method, strong pertinency, strong protective function, good immunological effect and low cost of the used material, and is suitable for mass production. The invention provides a practical method for preventing and curing the bacteremic septicemia of carps.
Owner:张秀军 +2
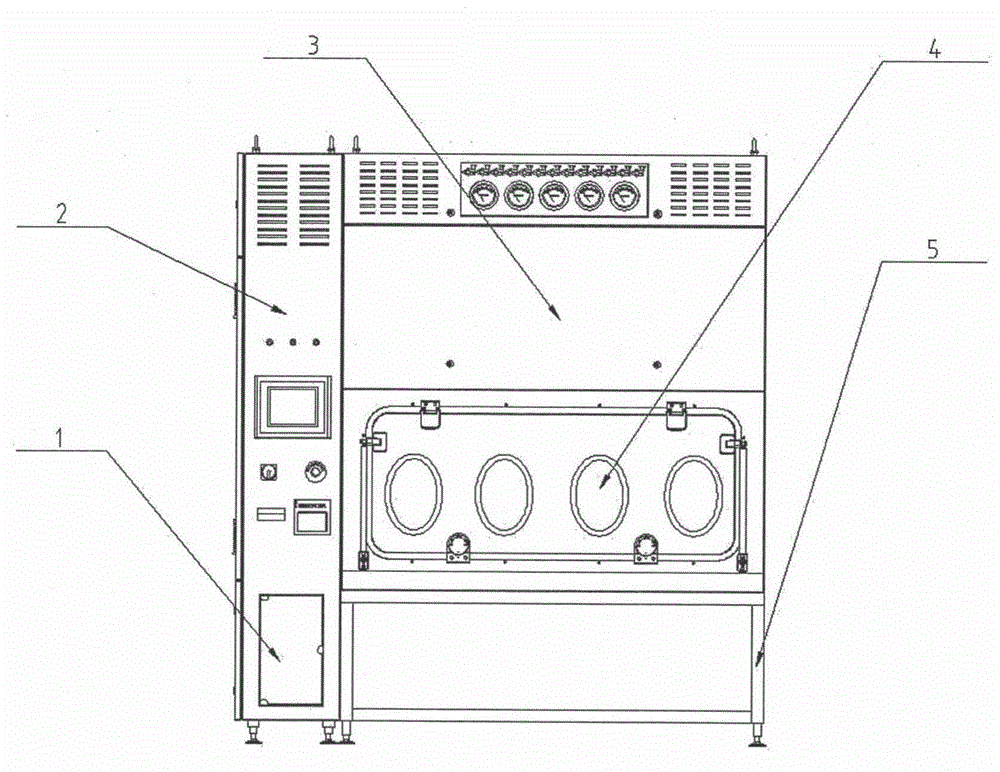
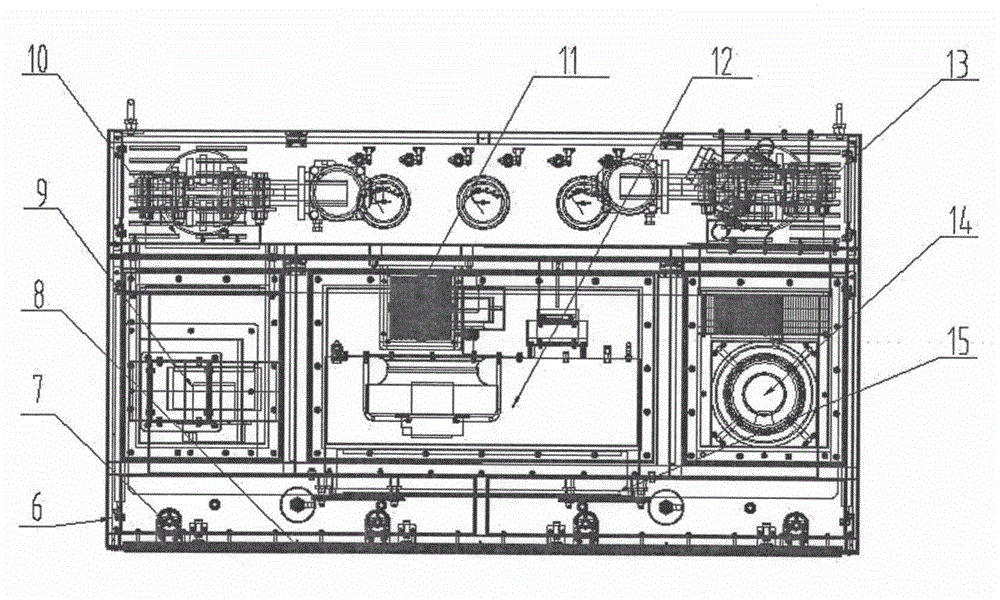
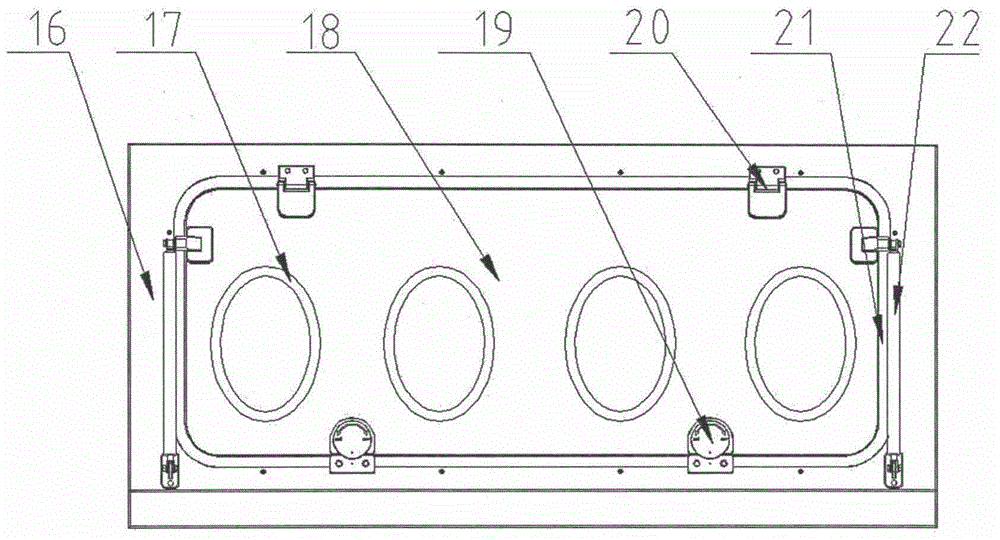
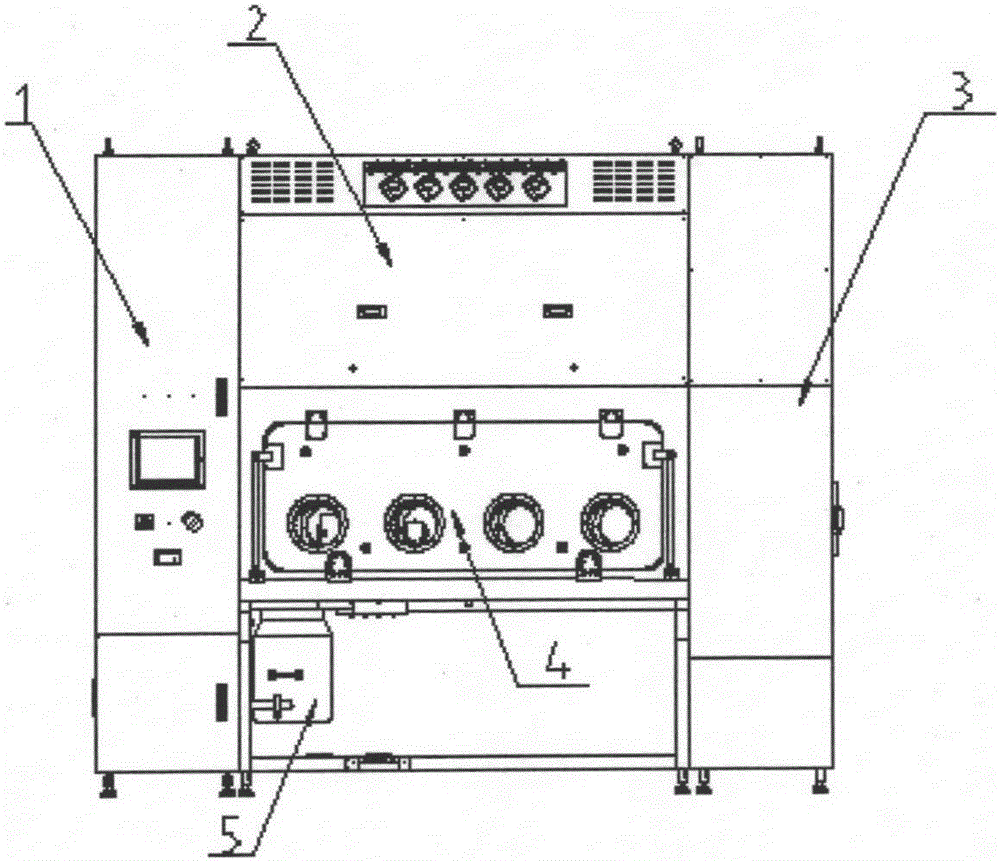
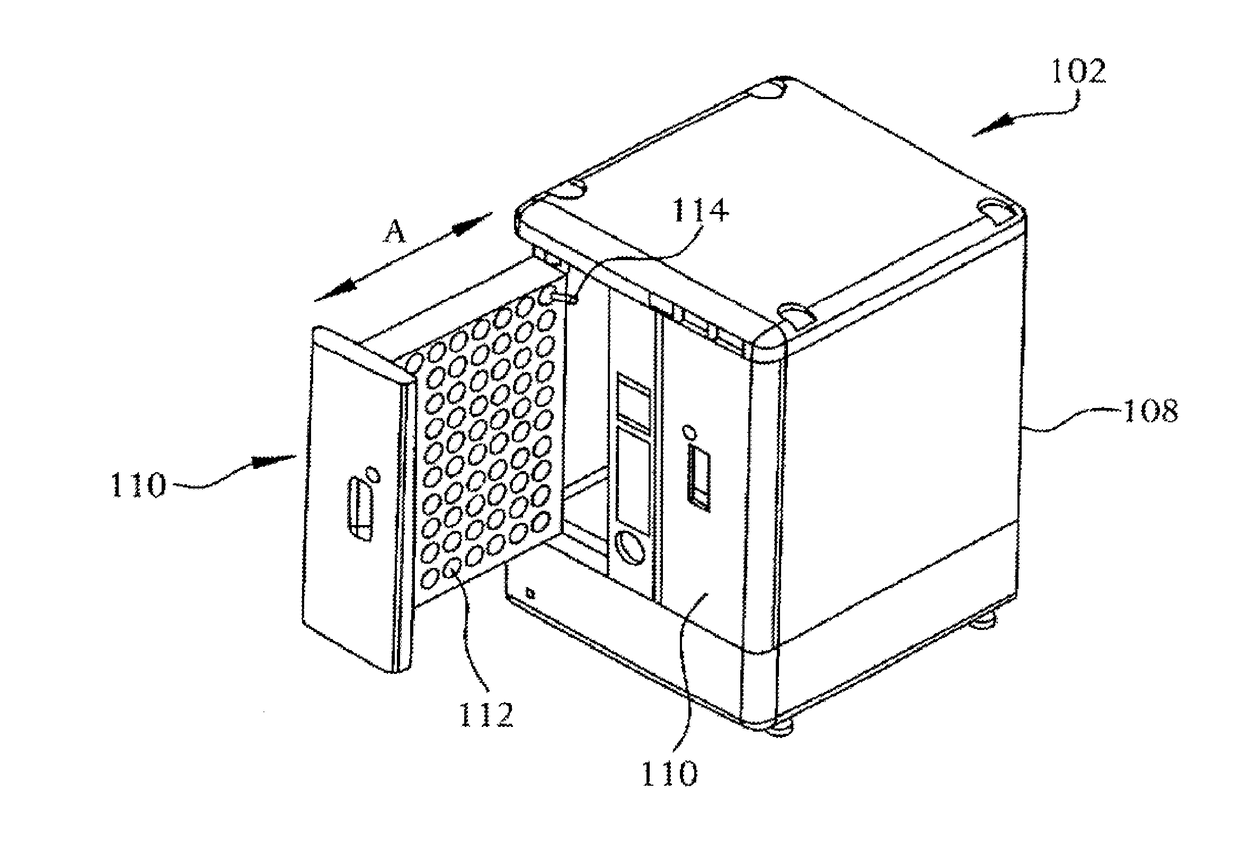
Sterility test process isolator
InactiveCN104353095AGuaranteed tightnessConvenience guaranteedManipulatorChemicalsOperating pointEngineering
The invention discloses a sterility test process isolator which is characterized by comprising a frame component at the bottom, a glove operation box component arranged on the frame component, an air handling system at the top and an electric system, wherein a hydrogen peroxide steam sterilization system is integrated on one side of the electric system. The isolator completely isolates internal production environment from operators and external environment, reduces the risk of artificial pollution and meanwhile reduces pollution to the operators and the external environment; the isolator reduces a production cleaning area, greatly increases the controllability of a production area and further lowers the operating cost and the maintenance cost; besides, the integrated hydrogen peroxide steam sterilization system is arranged, so that the on-line SIP is realized and the sterility of operating environment is guaranteed.
Owner:上海东富龙爱瑞思科技有限公司 +1
Busulfan injection and preparation method thereof
ActiveCN102151257AImprove stabilityExtended shelf lifeOrganic active ingredientsPharmaceutical delivery mechanismOrganic acidAlkaline earth metal
The invention provides a busulfan injection. Busulfan is dissolved in a mixed solvent of N,N-dimethyl acetamide with a volume ratio of 33% and polyethylene glycol 400 with a volume ratio of 67%, wherein the concentration of the busulfan is 6mg / ml, and the mixed solvent contains organic acid and alkali metal or alkaline earth metal salt of organic acid, which account for 0.01-5%w / v of the total amount of the mixed solvent; and the organic acid contains more than two carboxyl groups. The stability of main constituents of the busulfan injection provided by the invention can be improved remarkably so that the quality guarantee period of products is prolonged to more than 24 months; and various indices (such as appearance, content of tetrahydrofuran, content of main constituents, content of impurities, endotoxin, sterility test and the like) conform to the requirement of the quality standard. The active constituents and the auxiliary materials of the injection provided by the invention have no injection stimulation, and the injection can be used for clinical injection and can ensure that the medication safety cannot be lowered while the stability of products is improved.
Owner:SICHUAN CREDIT PHARMA +1
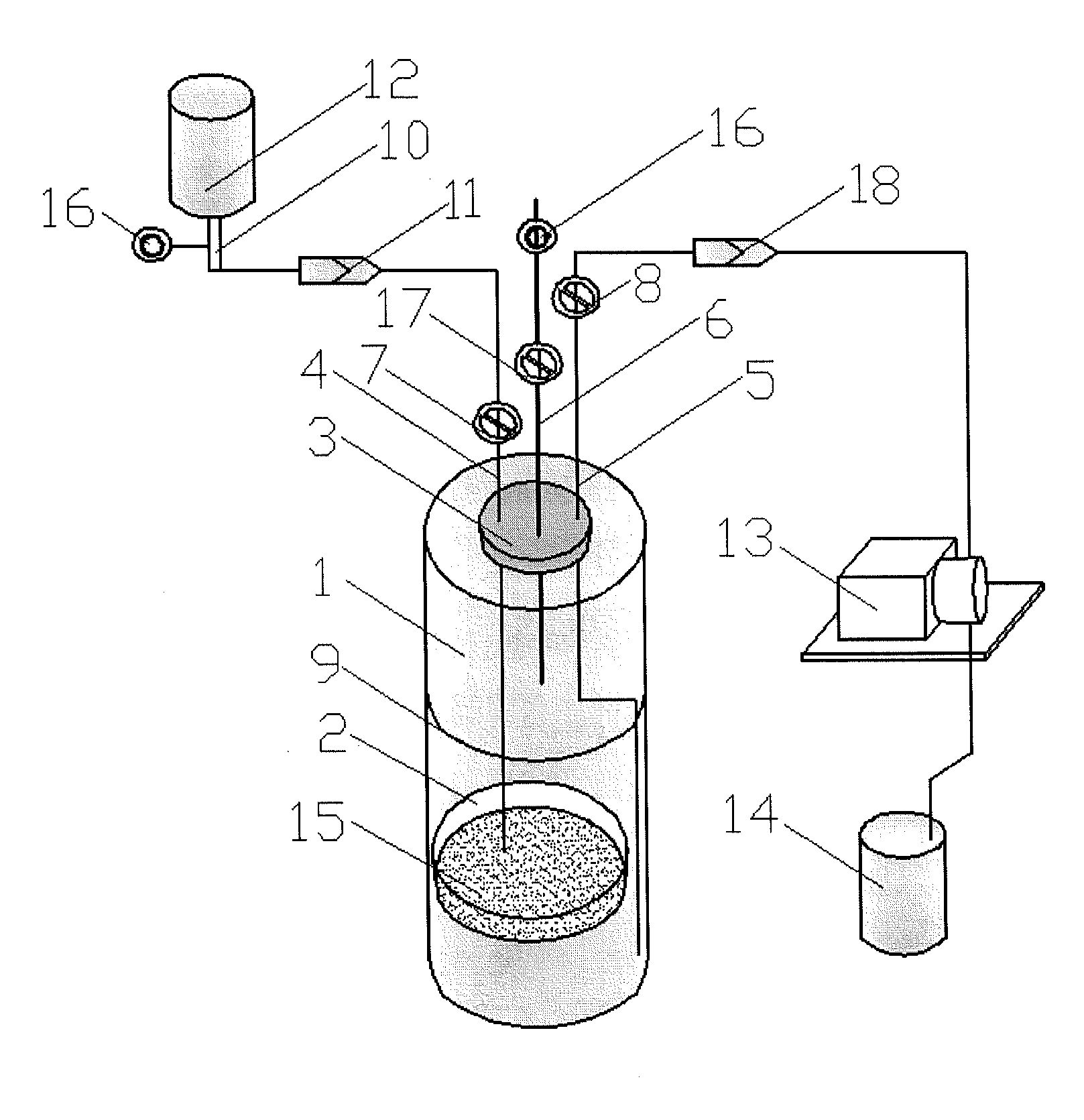
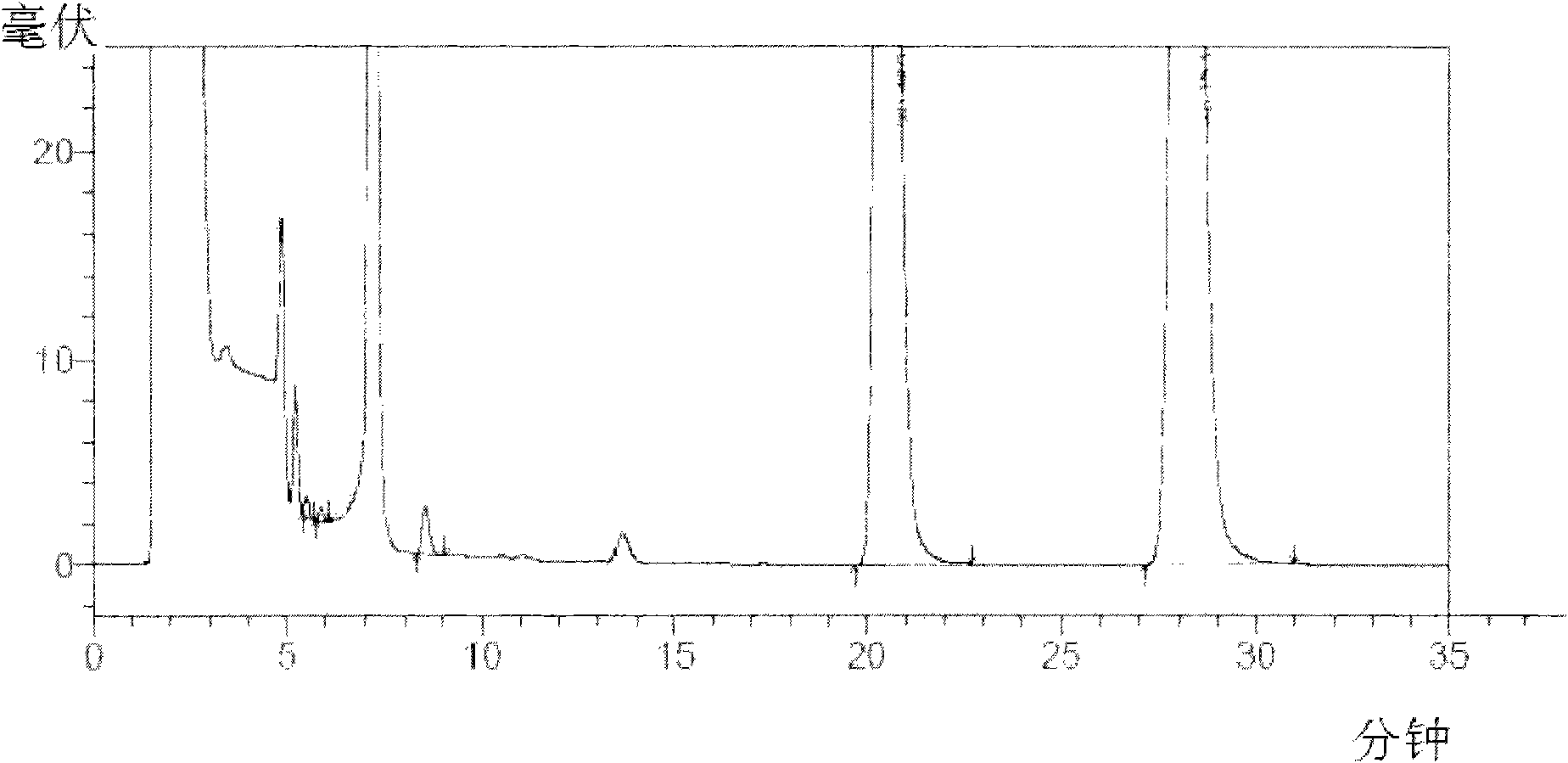
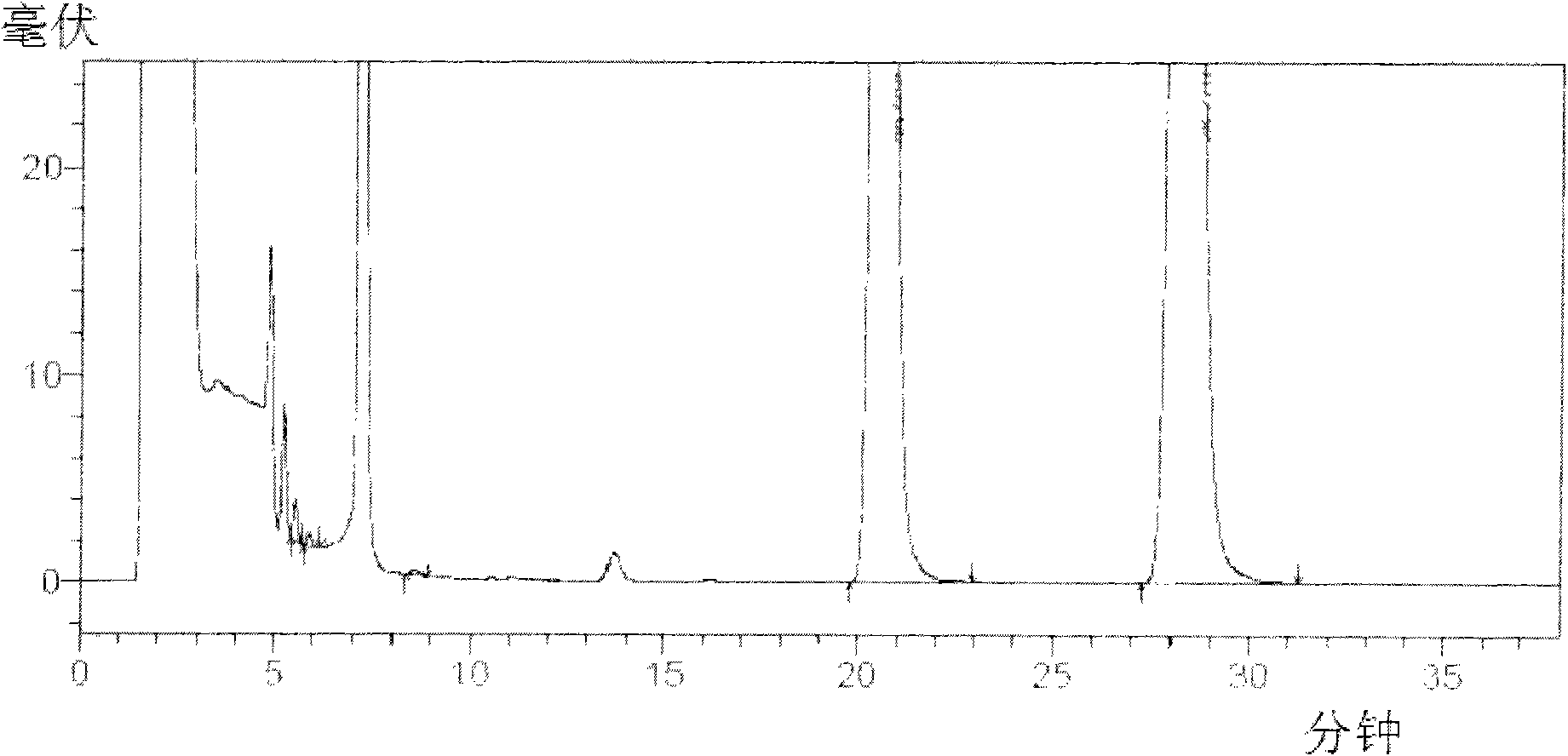
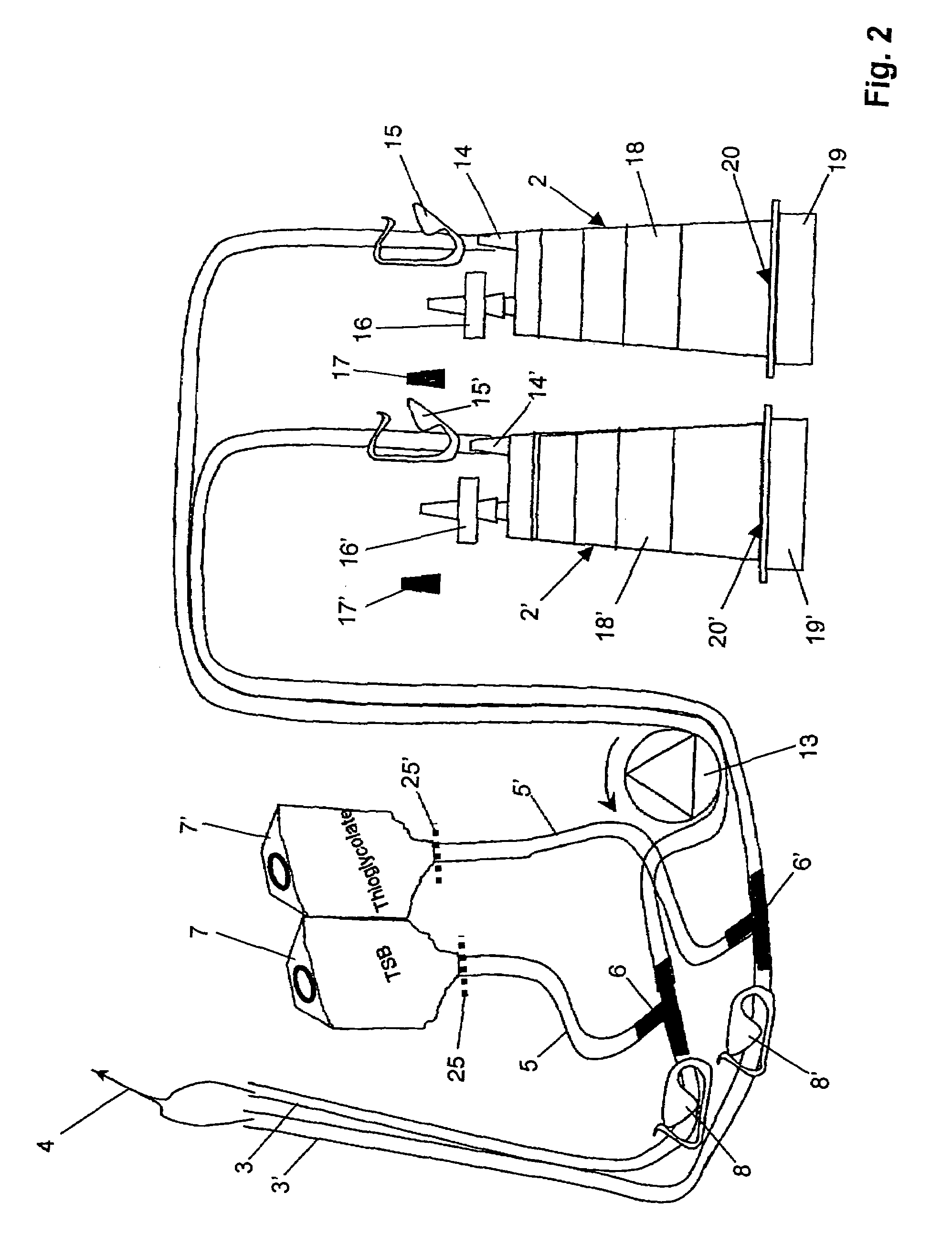
Device and method for sterility testing
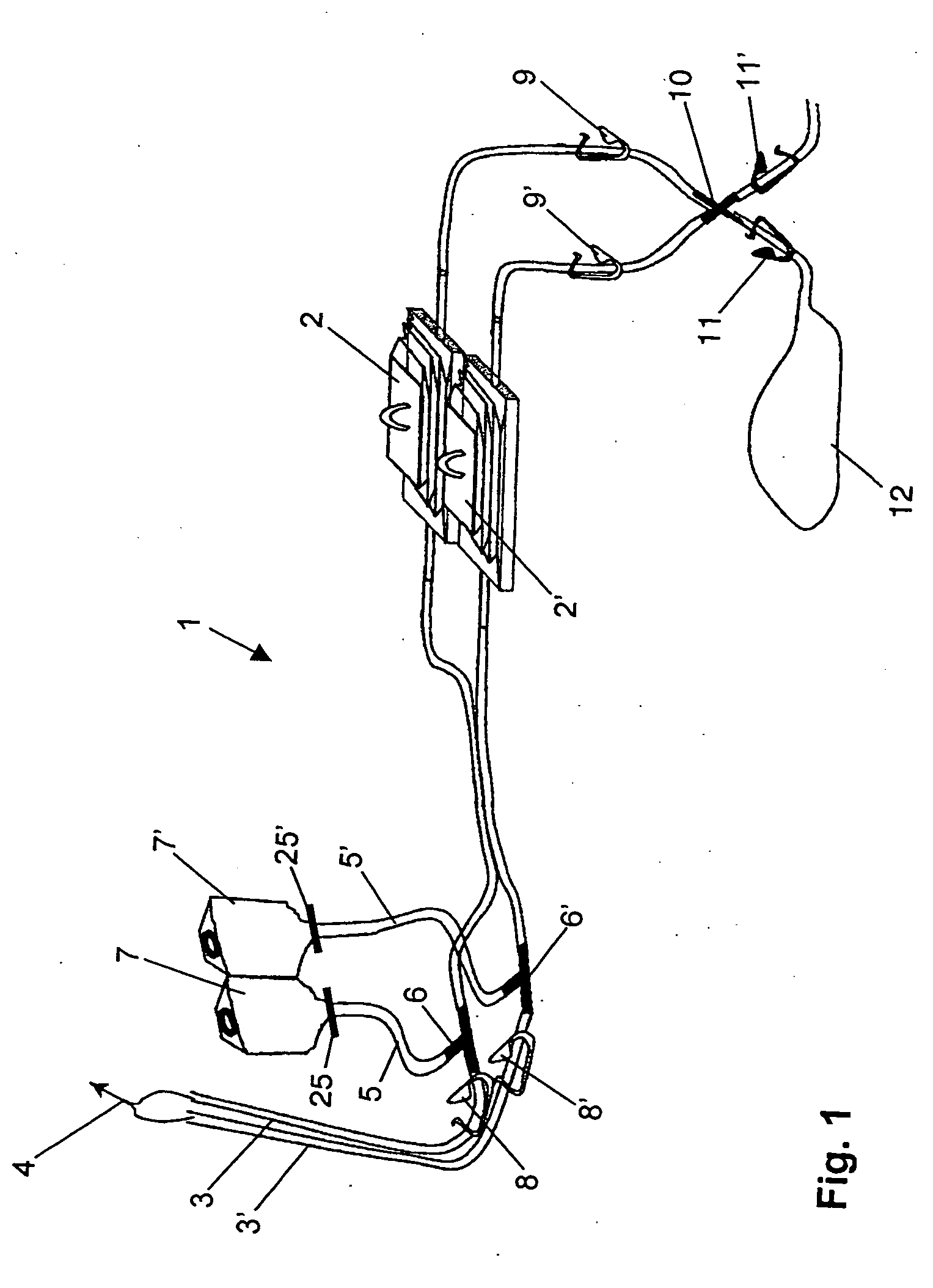
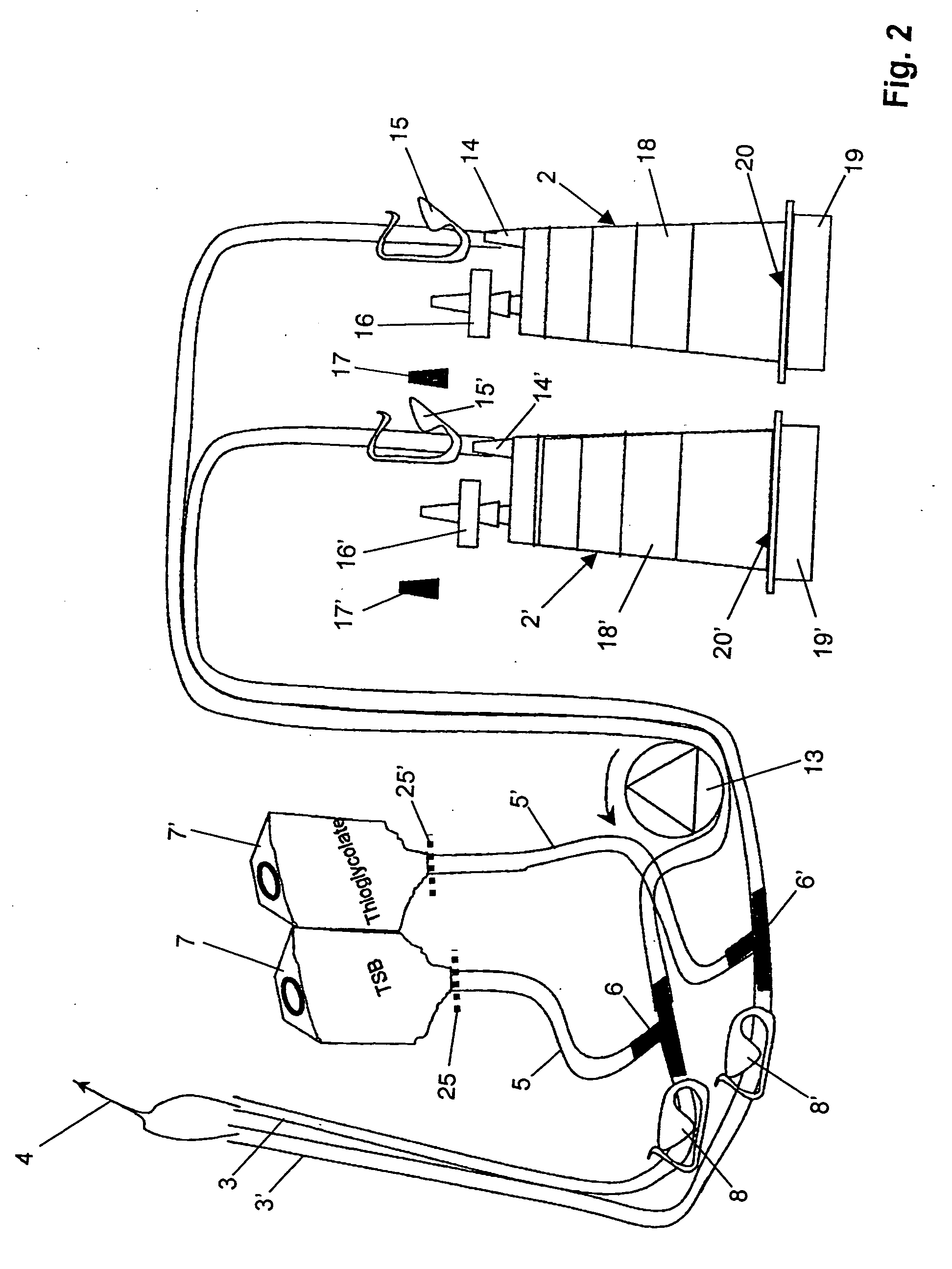
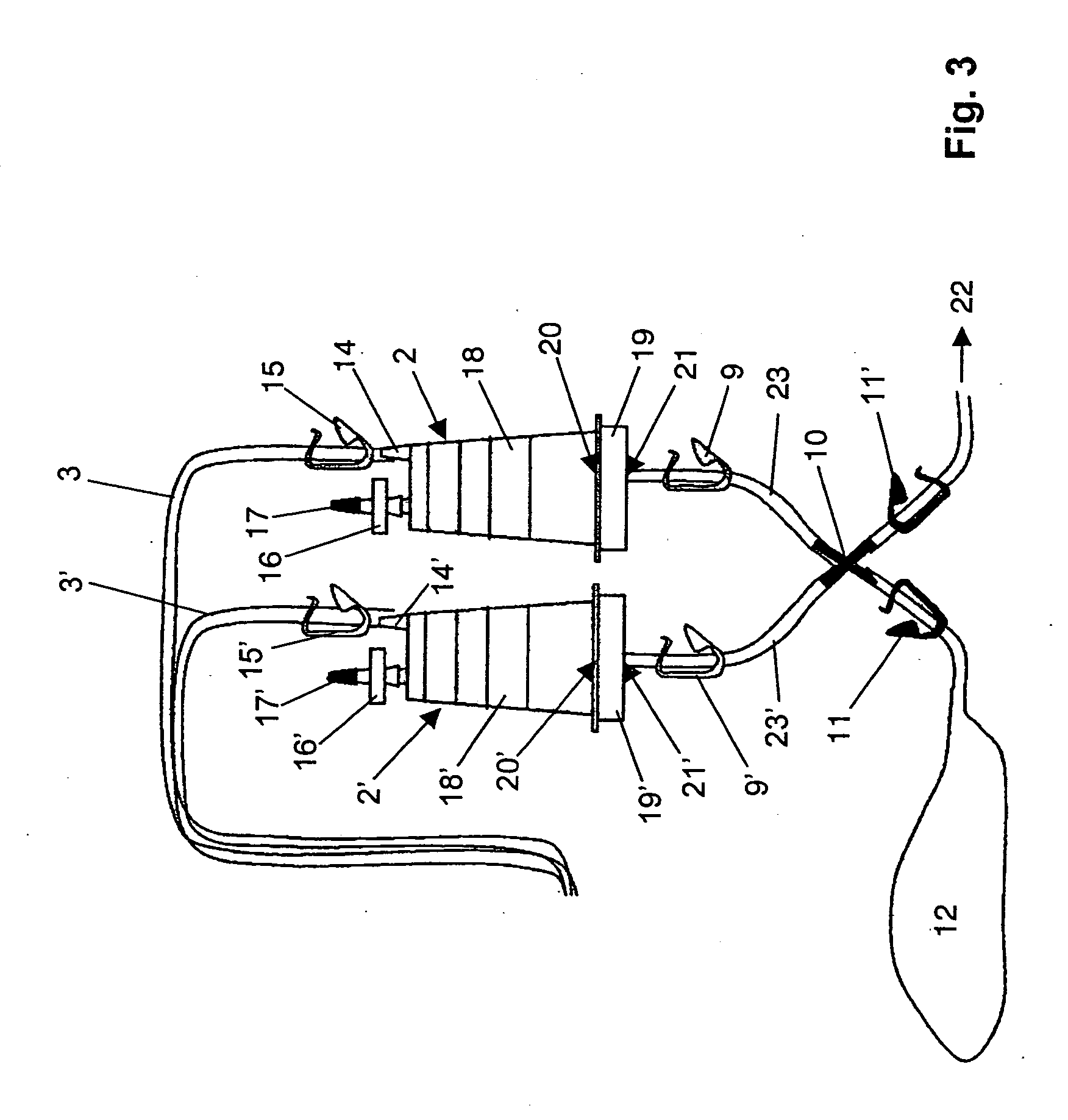
ActiveUS7354758B2Reduce time expenditureReduce riskBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringSterility test
Device and method for sterility testing, in particular of pharmaceutical products, includes a closed sterility test system having at least two test containers, in each case having an integrated test filter, an outlet connecting branch arranged below the test filter and an inlet connecting branch which is arranged above the test filter and can be connected with a tube connection via a sampling device to a sample container. The tube connection of the test container in each case has a distributing component via which a fixed tube connection to an associated nutrient medium container is formed and can be used to feed an associated nutrient medium to the test container.
Owner:SARTORIUS STEDIM BIOTECH GMBH
Coconut wine and brewing method thereof
InactiveCN102181351AIncrease added valueSimple processAlcoholic beverage preparationYeastAdditional values
The invention discloses the technical field of brewing, and relates to coconut wine and a brewing method thereof. The method comprises the following steps: taking coconut water; and adding SO2 into the coconut water; placing the mixture into a fermentation tank; taking supernatant after adjusting alcoholic strength and inoculating yeast for fermentation; adding SO2 in the supernatant; adjusting the alcoholic strength; adding bentonite; standing and filtering to obtain a rough product of the coconut wine; sealing and storing the rough product; adjusting the alcoholic strength; reducing the acidity; blending the flavor; clarifying and filtering the rough product subjected to refrigeration; degerming and filtering; identifying at low temperature and carrying out sterility test; and packaging to obtain the finished product coconut wine. The brewing method of the coconut method provided by the invention has the advantages of simple process, low cost and environmental friendliness, and can be used for effectively improving the additional value of the coconut, and is beneficial to promoting the development of the coconut and relevant industries in China; and the coconut wine has rich coconut aroma, conforms to the national edible wine stand, is a wine product with tropical amorous feelings, and has realistic significance.
Owner:HAINAN UNIVERSITY
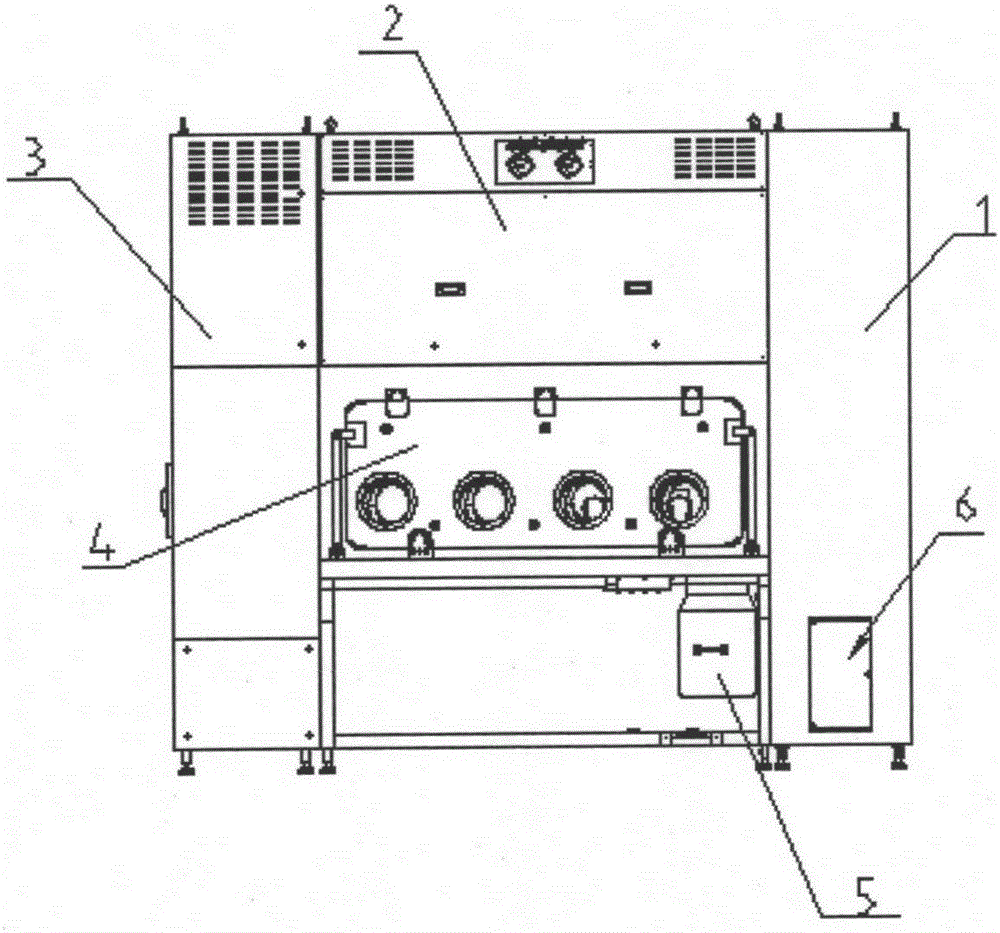
Sterility test process isolator for double-sided laminar flow operation
InactiveCN105196313AAchieve isolationNo harmGaseous substancesManipulatorAir treatmentProcess engineering
The invention discloses a double-sided sterility test process isolator. The isolator is characterized by comprising a glove bin system and an electrical cabinet system, wherein an air treatment system is arranged at the top of the glove bin system; the glove bin system comprises a bin body; a glove operation component is arranged on the bin body, and comprises operation window sealing glass; and gloves are connected with the operation window sealing glass by virtue of a glove connection component. The sterility test process of sterile medicines can be carried out under a controlled environment all the time by virtue of the sterility test isolator, so that toxic substances inside the sterile medicines cannot hurt operators, pollutants in the external environment are prevented from entering the operation space and polluting to-be-tested products, and the requirement of a production region for the cleanness of a background environment can be reduced. Changes of an arbitrary environment parameter inside the sterility test process isolator can be correspondingly regulated and controlled, so that isolation of the sterility test step after production of sterility medicines can be realized.
Owner:上海东富龙爱瑞思科技有限公司 +1
Improvements in using blood culture platforms for commercial sterility tests
ActiveUS20170191110A1Reduce parameterMicrobiological testing/measurementTesting dairy productsMicroorganismBottle
A system that indicates the presence or absence of microorganisms in fluid food products. The system has a bottle for receiving sample to be tested. The bottle has a sensor that will monitor and detect changes in at least one sample parameter, but no additives that contain nutrients that support microbial growth. The bottle is placed in an incubator and the sensor in the bottle is monitored for changes. The incubator is programed so that, if the sensor detects that the value of the monitored parameter has reached a certain value, then the sample is determined to be positive for microbial growth.
Owner:BECTON DICKINSON HLDG
Novel trivalent inactivated vaccine for swine streptococcosis
ActiveCN101721695AOvercoming deficiencies in infectionAchieve the effect of multiple defenses with one injectionAntibacterial agentsBacterial antigen ingredientsAntigenLancefield grouping
The invention discloses a novel trivalent inactivated vaccine for swine streptococcosis, which is prepared by C55138 antigen of streptococcus equi subsp zooepidemicus of a lancefield group C, C55914 antigen of streptococcus of a lancefield group D and C55949 antigen of streptococcus of a lancefield group E through the steps of strain propagation, bacterium liquid culture, purity check, viable count, inactivation, detection of inactivation, precipitation and concentration, removal of toxins, mixing for hybridization, sterility test of vaccines, safety inspection of the vaccines, efficiency test and split charging. The novel trivalent inactivated vaccine of the swine streptococcosis prepared by the invention can prevent three types of swine streptococcosis caused by the streptococcus of thelancefield group C, the lancefield group D and the lancefield group E simultaneously and has wide application prospect.
Owner:广东永顺生物制药股份有限公司
Separation and purification method of endophytic fungi of solidago canadensis
InactiveCN102690759AObvious growth effectGrowth inhibitory effectFungiMicroorganism based processesBiotechnologyAntibacterial activity
The invention relates to a separation and purification method of endophytic fungi of solidago canadensis. The method comprises the following steps of: 1, collecting stems and leaves of the solidago canadensis; 2, carrying out solid culture and sterility test on the stems and the leaves; and 3, carrying out isolated culture on the endophytic fungi of tissues of the solidago canadensis to obtain the endophytic fungi of the solidago canadensis. A fungus colony has silk velvet texture and a villous surface, does not generate pigment and is light olivaceous and black brown at the back; and the gene complex of the endophytic fungi ITS of the solidago canadensis is composed of 602 basic groups (bp). The geomicrobiology status of a bacterial strain of chaetomiumglobosumyt03 with broad-spectrum antibacterial activity is determined, and the endophytic fungi is found to have relatively remarkable role in inhibiting mycelial growth of six provided plant pathogenic fungi through antibacterial test of the fermentation liquid of the chaetomiumglobosumyt03.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Preparation method for microencapsulated oral live vaccine of gosling plague
The present invention discloses a preparation method for a microencapsulated oral live vaccine of gosling plague. The preparation method comprises: preparing a gosling plague SYG strain into a vaccine half finished product by goose embryo proliferation culture; adding a 5% sucrose and skimmed milk solution after a sterility test is qualified, and uniformly stirring; adding 20-40% porous starch tothe half finished product solution, stirring for 20-40 minutes at a certain temperature, wherein the temperature is controlled to 37 DEG C; mixing the resulting liquid and a 1-2.5% sodium alginate solution, completely stirring, and then carrying out dehydration and drying by a fluidized bed to prepare the microencapsulated vaccine dry powder of the gosling plague, wherein the volume of the resulting liquid is the same as the volume of the sodium alginate solution. The method of the present invention ha characteristics of science, simpleness, stable and reliable production, less loss of vaccine titer. With adopting the vaccine microencapsulation technology, the live vaccine of the gosling plague can be adopted for immunization by the oral route, the stress reaction is reduced, the sustained release function is provided for the live vaccine of the present invention, and the breeder goose in the laying period can use the live vaccine as usual.
Owner:HANGZHOU JIANLIANG VETERINARY BIOLOGICAL PREPARATIONS CO LTD
Human sperm cryoprotectant containing D-trehalose and preparation method of human sperm cryoprotectant containing D-trehalose
InactiveCN105746494ARaise the ratioIncrease movement speedDead animal preservationWater bathsGlycerol
Owner:山西省人口计生委科学研究所
Chicken egg yolk antibody resisting human A rotavirus as well as preparation method and application thereof
InactiveCN103554254AClear genetic backgroundMature and perfect structureEgg immunoglobulinsImmunoglobulins against virusesAntigenRotavirus RNA
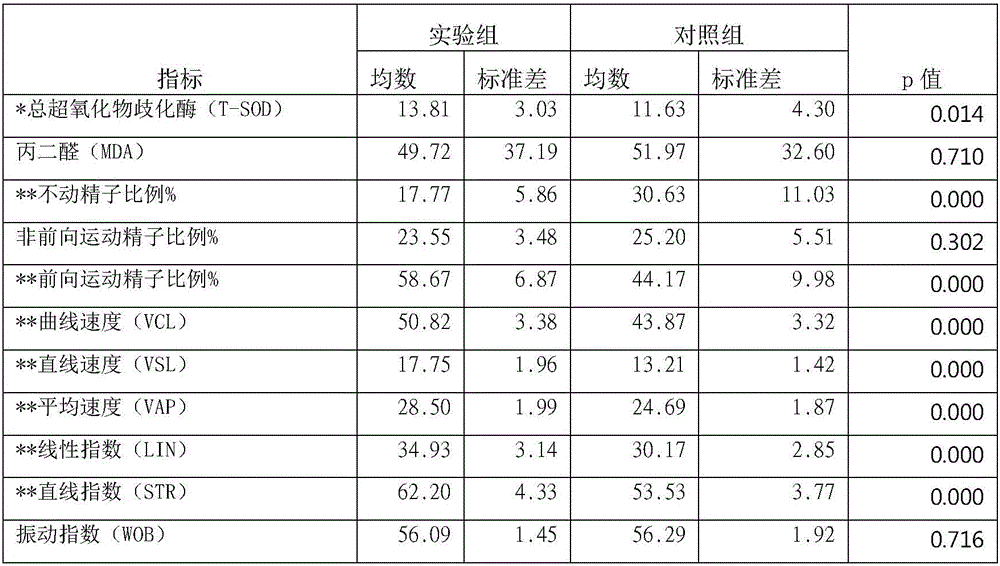
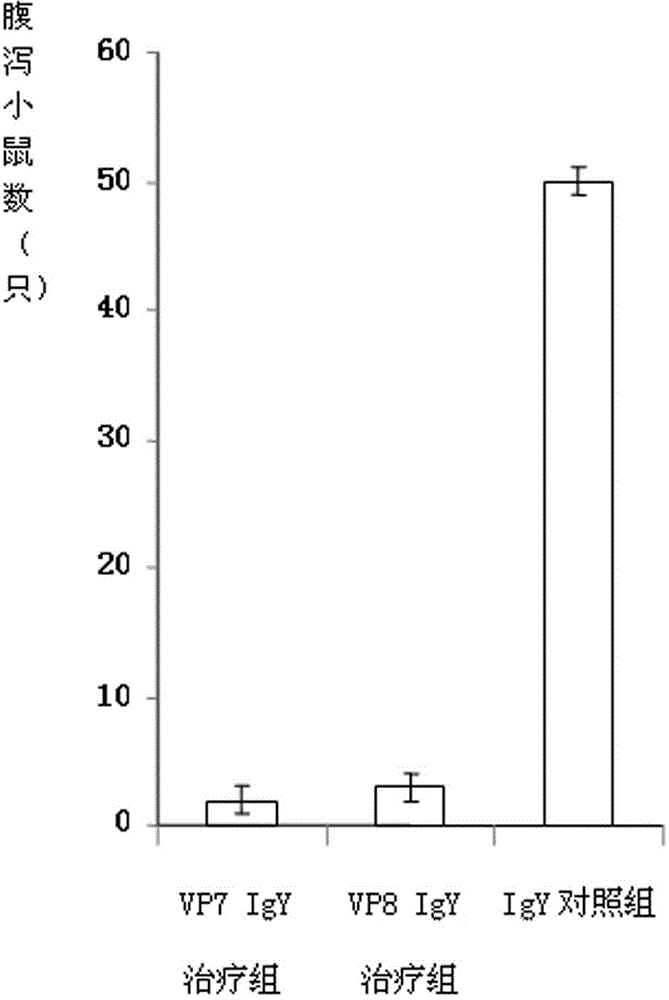
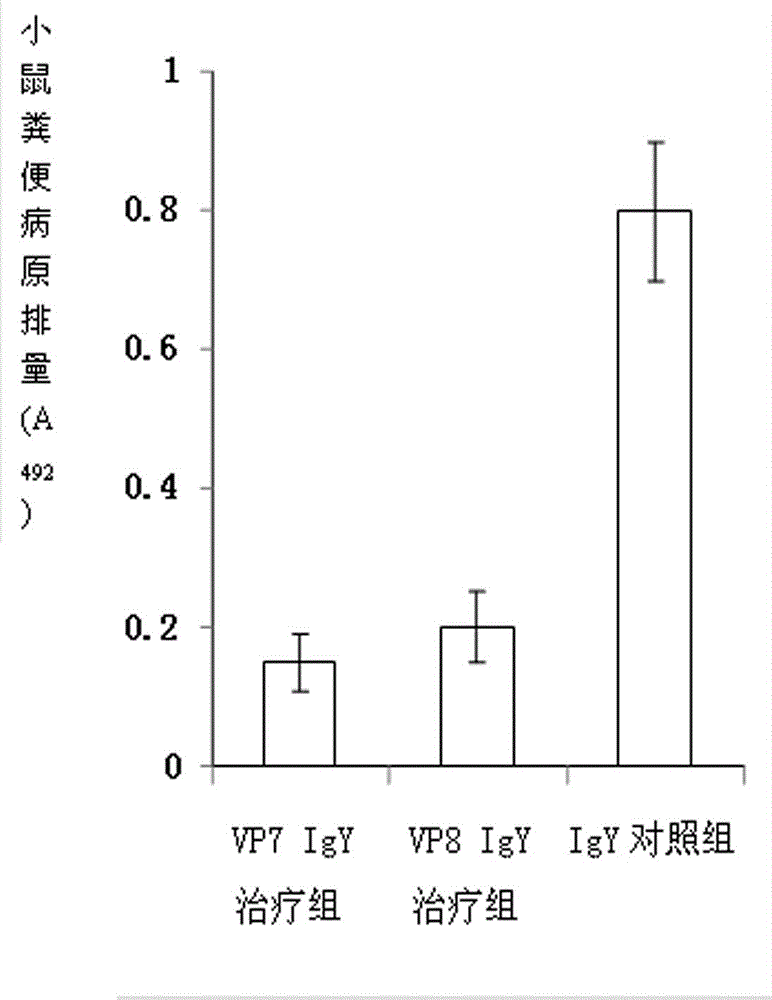
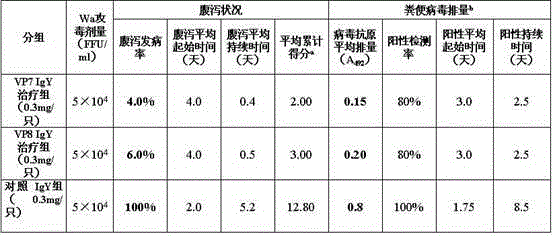
The invention discloses a chicken egg yolk antibody resisting human A rotavirus as well as a preparation method and application thereof. The method comprises steps of expressing and purifying structural protein VP7 or VP8 of human rotavirus in vitro, immunizing laying hens, collecting eggs, and separating and purifying egg yolk antibody of VP7 or VP8. The prepared chicken egg yolk antibody can be freeze dried and prepared into antibody dry powder for preventing and treating rotavirus. The preparation method has the beneficial effects that a large quantity of VP7 and VP8 antigens are expressed and recombined in vitro by a genetic engineering technology, the benefits are high, the cost is low, small field is required, and the method is environment-friendly; the potential risk of scattering virus caused by inactivation by rotavirus or attenuated vaccine in the traditional preparation process of egg yolk antibody is avoided, and the method meets the requirement of animal welfare. The egg yolk antibody prepared by the method can be prepared into oral antibody dry powder biological agent after sterility test, has obvious effect in treating rotavirus infectious diarrhea, takes effect rapidly and has low cost, and the prevention rate reaches over 95%.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
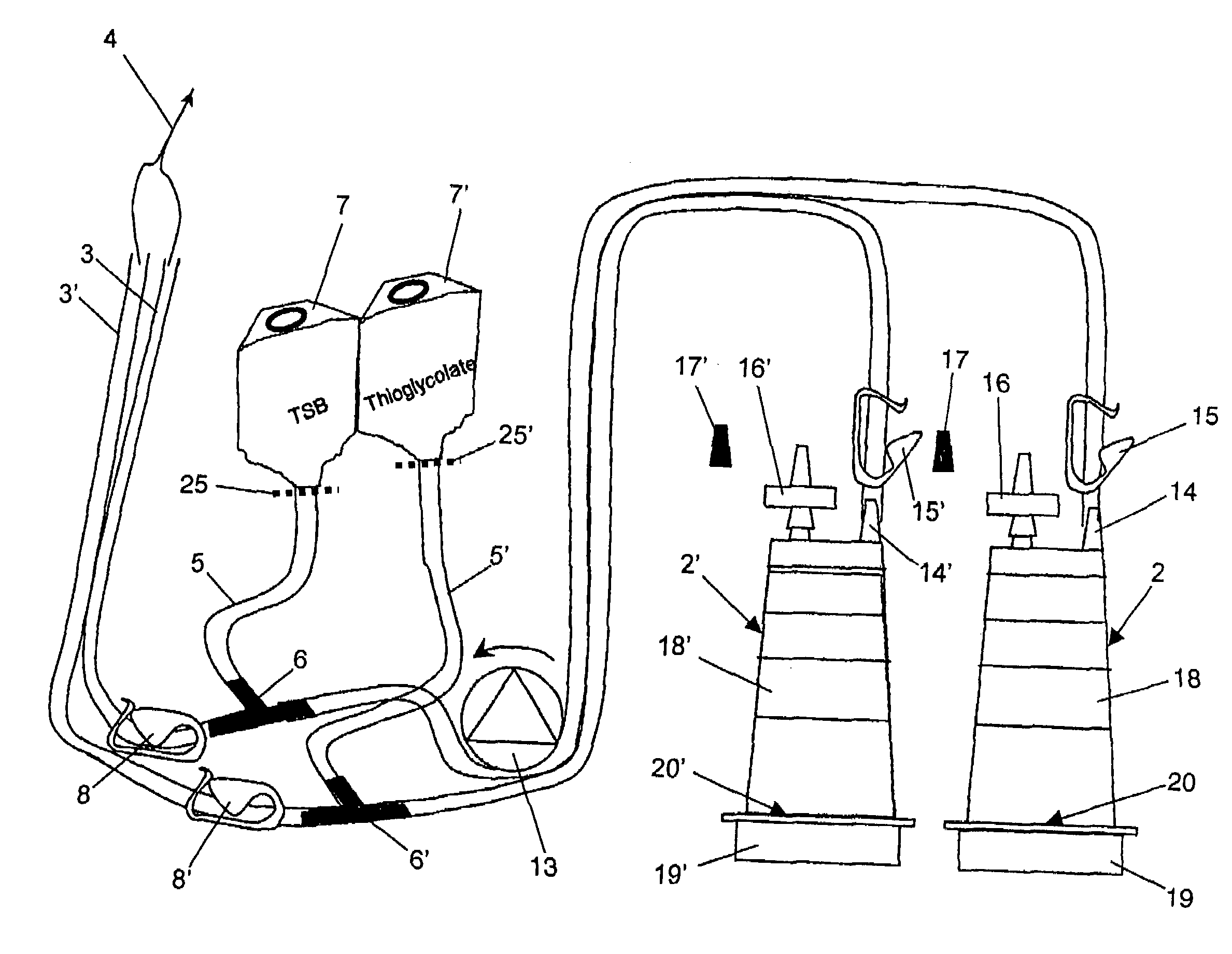
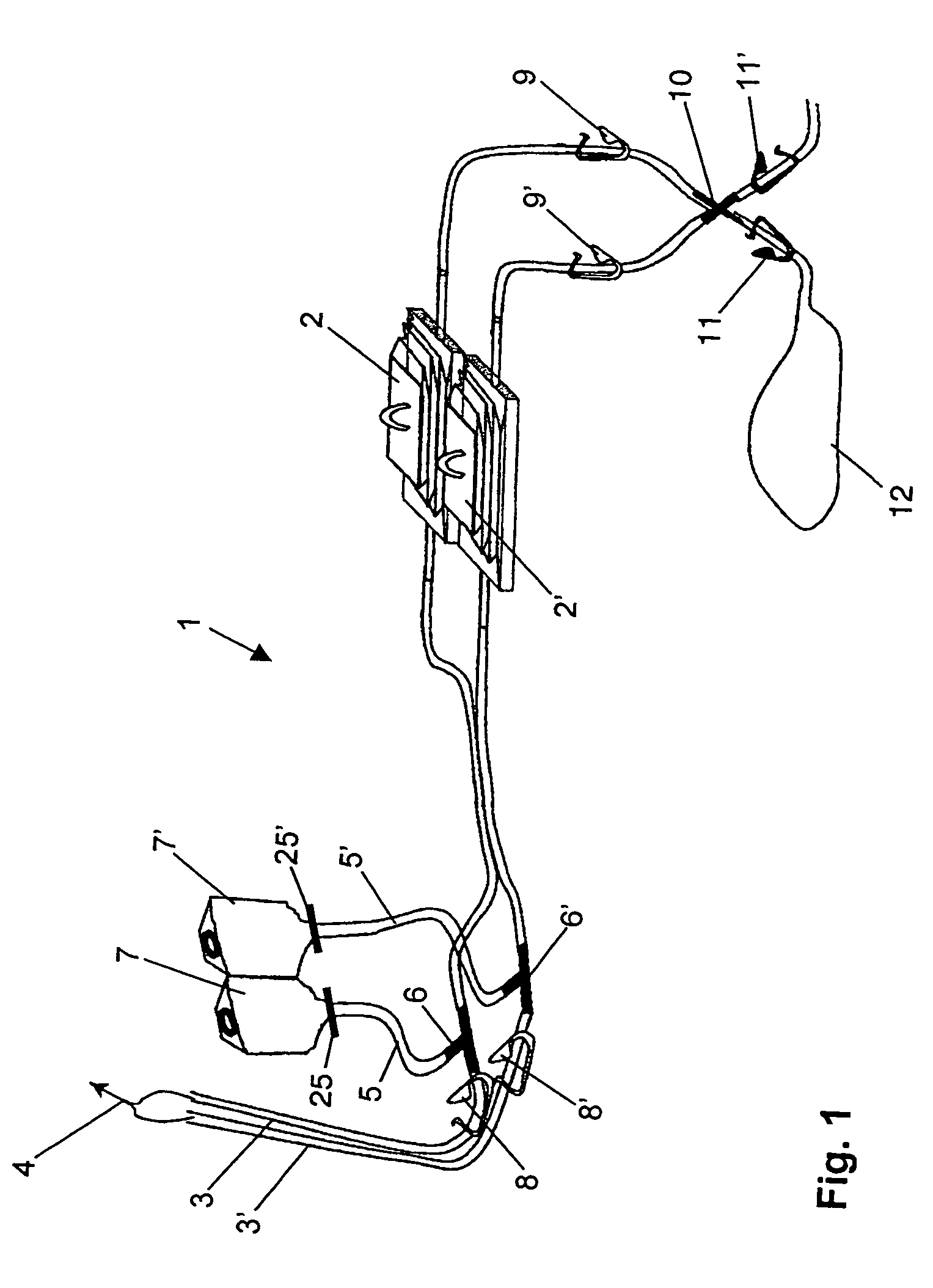
Device and method for sterility testing
ActiveUS20050048598A1Reduce riskReduce time expenditureBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringSterility test
Device and method for sterility testing, in particular of pharmaceutical products, includes a closed sterility test system having at least two test containers, in each case having an integrated test filter, an outlet connecting branch arranged below the test filter and an inlet connecting branch which is arranged above the test filter and can be connected with a tube connection via a sampling device to a sample container. The tube connection of the test container in each case has a distributing component via which a fixed tube connection to an associated nutrient medium container is formed and can be used to feed an associated nutrient medium to the test container.
Owner:SARTORIUS STEDIM BIOTECH GMBH
Fast dissolution preparation method for blood culture medium
The present invention relates to the technology field of the biomedicine, in particular to a preparation method for the rapid dissolving of a blood culture medium. Firstly, the raw materials of the culture medium are weighed, and the raw materials include that peptone is 10 g, beef extract is 3 g, sodium chloride is 5 g, sodium citrate is 3 g, and agar is 0.5 g; the fast solution is prepared by the preparation method that 24.6 percent of magnesium sulfate solution is prepared to be used as fast solution 1 to be reserved; 0.5 percent of paraaminobenzoic acid solution is prepared to be used as fast solution 2 to be reserved; the mixing is realized by that about 20 ml raw material of the fast solution is taken and 10 ml fast solution is taken to be added into the raw material of the culture medium and agitated into paste, and then 1000ml distilled water is added into to be rocked and mixed, and statically positioned for three to five minutes to obtain the transparent culture medium fluid; the pH value is adjusted to be 7.4; the culture medium fluid is subpackaged in a sealed liquid bottle, and then sterilized in the high pressure; the finished product of the blood culture medium includes the obtaining process that 100 units of penicillase are added in each bottle through an injection syringe or not added, after the sterility test, the blood culture medium is reserved in the room temperature. Compared with the prior art, the present invention eliminates the long time heating dissolving and filter process, saves the preparation time and the filter material, and the operation issimple and convenient.
Owner:云南迪安医学检验所有限公司
Preparation method using bacterial lysate as inactivated vaccine adjuvant
PendingCN114028558ABiosafetyImprove immunityRotary stirring mixersTransportation and packagingBiotechnologyBacilli
The invention relates to a preparation method using bacterial lysate as inactivated vaccine adjuvant. The preparation method comprises the following steps: step 1, respectively putting corynebacterium pseudotuberculosis and rhodococcus equi into an LB culture medium for culturing at 37 DEG C, and harvesting thalli through a centrifugal machine; step 2, diluting each thallus to 8.0* 10<9> cfu / mL by using sterilized normal saline, and crushing the bacterial liquid by using a high-pressure homogenizer; and step 3, after sterility test, mixing the split products of corynebacterium pseudotuberculosis and rhodococcus equi with the two bacteria inactivated by formaldehyde according to a ratio of 1: 1 to obtain the inactivated vaccine added with the adjuvant. The preparation method has the characteristics of safety, high efficiency, strong immune enhancement capability and low cost, and is suitable for producing various bacterial inactivated vaccines.
Owner:ZHANGZHOU PIEN TZE HUANG PHARM +1
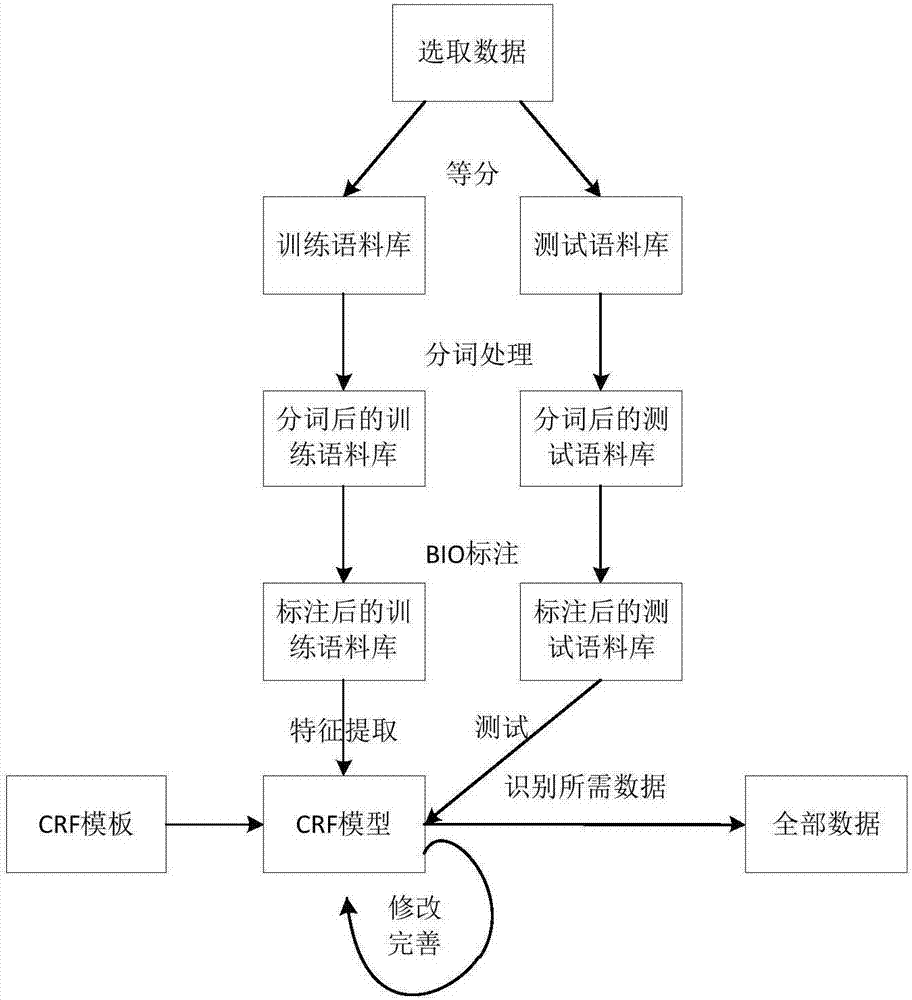
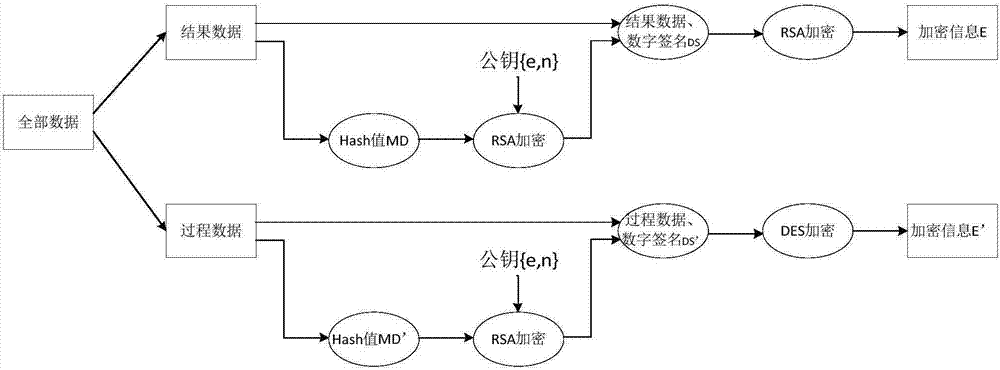
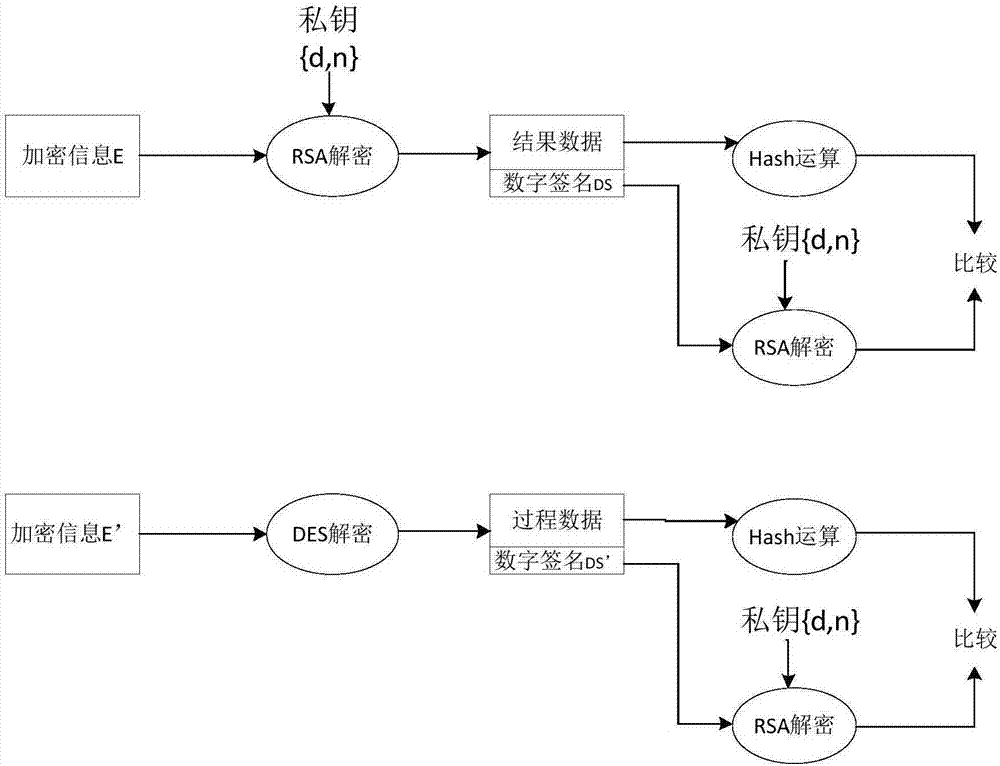
Sterility test data classification and encryption method based on conditional random field algorithm
InactiveCN106899572AWon't leakImprove securityMultiple keys/algorithms usageUser identity/authority verificationConditional random fieldDigital signature
Disclosed in the invention is a sterility test data classification and encryption method based on a conditional random field algorithm. The method comprises: first, marking important result data generated in a sterility test process, training a conditional random field model and recognizing the important result data; second, performing classification and encryption on the important result data recognized by the conditional random field model, and transmitting encrypted data to a receiver; and finally, recovering the data without loss by the receiver by virtue of a reversible decryption process. According to the method, the important result data generated in the sterility test process is recognized by using the conditional random field method, and the important result data is separated from process data for classification and encryption. At the same time, a digital signature technology utilized in the method can effectively prevent messages from being tampered so that the data cannot be leaked out. The sterility test data classification and encryption method can greatly reduce encryption time and improve the system efficiency while ensuring the security of the important data information.
Owner:ZHEJIANG UNIV
Sterility test method for antibiotic sustained-release pharmaceutical preparation
ActiveCN103421877ANo negative effects on growthRemove inhibitionMicrobiological testing/measurementMedicineCalcium Polycarbophil
The invention discloses a sterility test method for an antibiotic sustained-release pharmaceutical preparation, wherein a high-molecular polymer calcium polycarbophil is taken as a sustained-release matrix of the antibiotic sustained-release pharmaceutical preparation. The sterility test method comprises: employing a divalent cation metal salt as a precipitating agent to precipitate calcium polycarbophil in the antibiotic sustained-release pharmaceutical preparation, and further to have the antibiotic sustained-release pharmaceutical preparation filtered through a film, culturing the film, and determining whether the antibiotic sustained-release pharmaceutical preparation is sterile to realize sterility test. The sterility test method is applicable to sterility test on products which contain antibiotic and calcium polycarbophil as the sustained-release matrix, and cannot be filtered by employing the film.
Owner:QILU PHARMA CO LTD
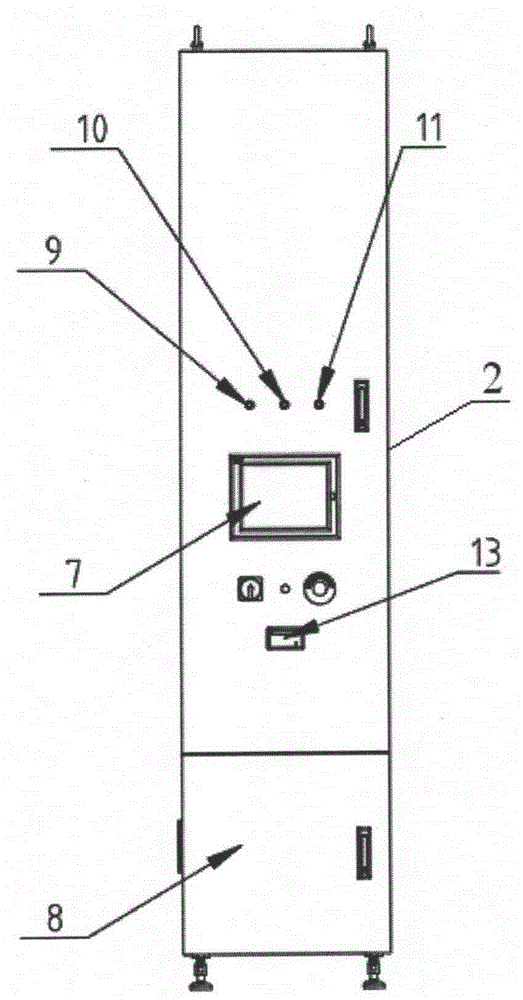
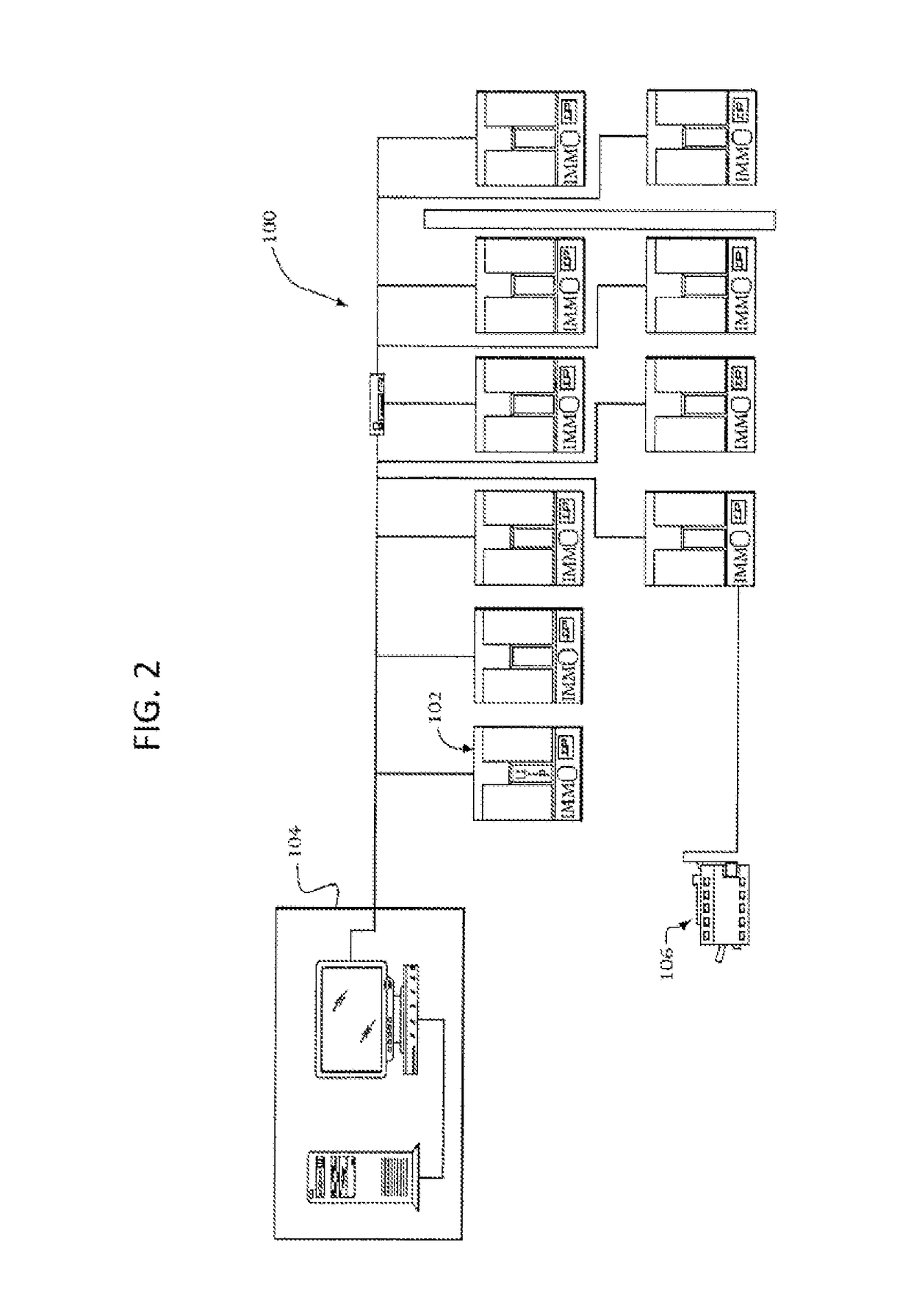
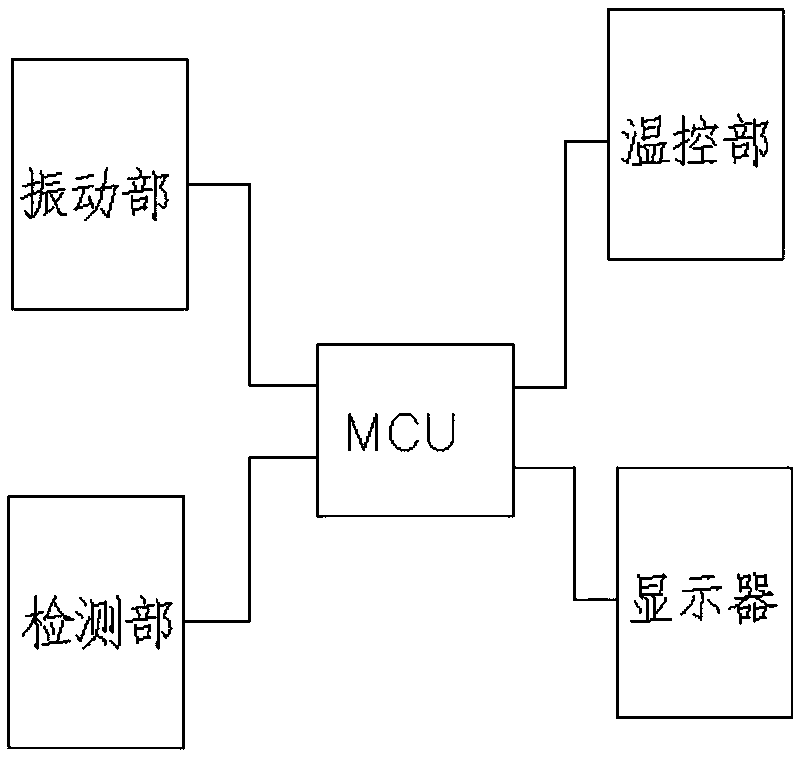
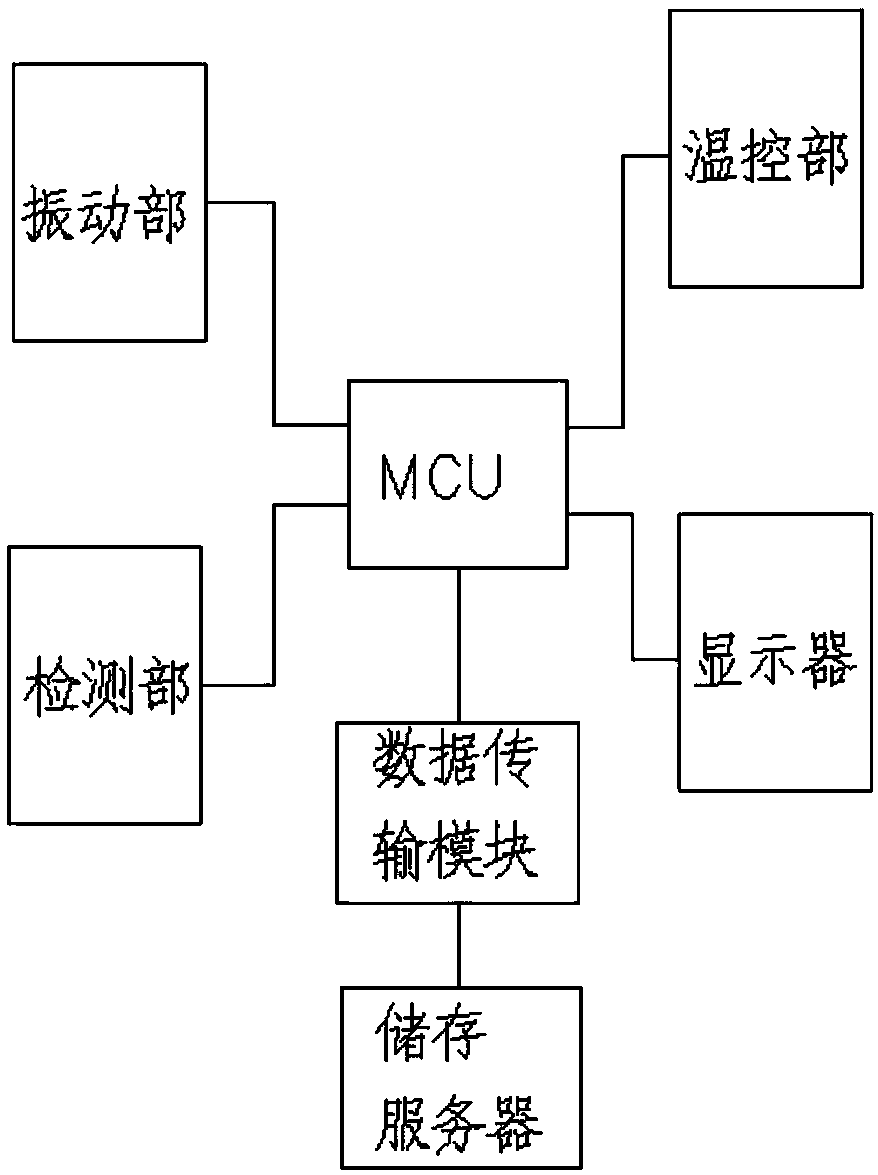
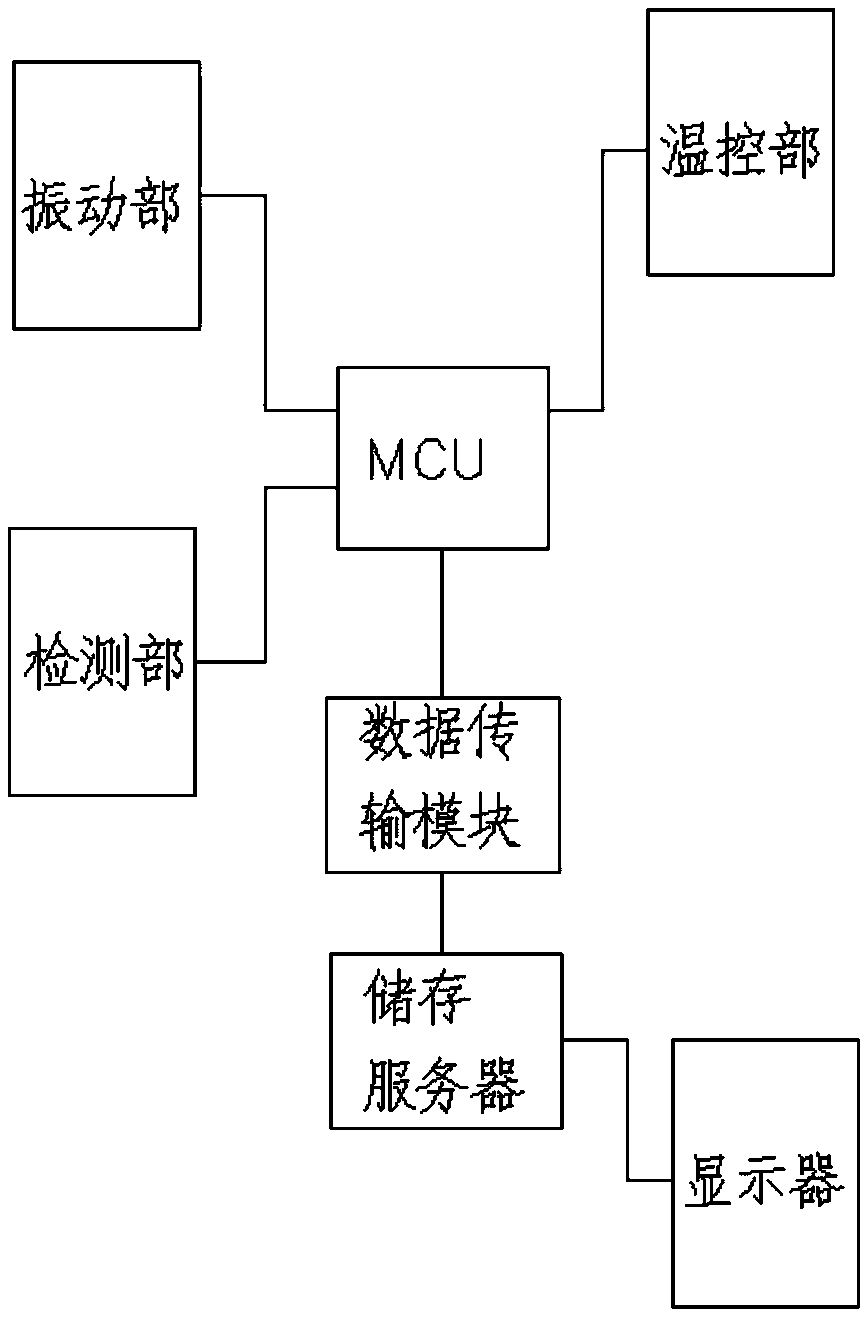
Sterile rapid detection system of medical equipment and application method thereof
PendingCN109306319AReduce usage timeImprove detection limitBioreactor/fermenter combinationsBiological substance pretreatmentsTemperature controlMedical equipment
The invention relates to a sterile rapid detection system of medical equipment. The sterile rapid detection system comprises an automatic detector for sterile test and a culture bottle, wherein the culture bottle is used for holding a culture medium and a sterilized biological indication bacteria tablet, the culture bottle is used for being arranged in the automatic detector for the sterile test,and the automatic detector for the sterile test is used for performing vibration culture on the culture bottle; the automatic detector for the sterile test also comprises a vibration part, a temperature control part and a detection part; and the vibration part, the temperature control part and the detection part are respectively connected with an MCU chip. The sterile rapid detection system can realize the automatic and rapid sterile monitoring of the medical equipment and the sterilized biological indication bacteria tablet and the like; and compared with the conventional detection means suchas an eye observation result in static culture, the detection limit can be improved, the detection period can be greatly shortened, the subjective determination error of the eye observation can be reduced, and the application time of a biochemical culture box can be shortened. At present, the study on the automatic rapid sterile detection technology of a medical equipment product is still in theblank.
Owner:山东省医疗器械产品质量检验中心 +1
Busulfan injection and preparation method thereof
ActiveCN102151257BImprove stabilityExtended shelf lifeOrganic active ingredientsPharmaceutical delivery mechanismOrganic acidAlkaline earth metal
The invention provides a busulfan injection. Busulfan is dissolved in a mixed solvent of N,N-dimethyl acetamide with a volume ratio of 33% and polyethylene glycol 400 with a volume ratio of 67%, wherein the concentration of the busulfan is 6mg / ml, and the mixed solvent contains organic acid and alkali metal or alkaline earth metal salt of organic acid, which account for 0.01-5%w / v of the total amount of the mixed solvent; and the organic acid contains more than two carboxyl groups. The stability of main constituents of the busulfan injection provided by the invention can be improved remarkably so that the quality guarantee period of products is prolonged to more than 24 months; and various indices (such as appearance, content of tetrahydrofuran, content of main constituents, content of impurities, endotoxin, sterility test and the like) conform to the requirement of the quality standard. The active constituents and the auxiliary materials of the injection provided by the invention have no injection stimulation, and the injection can be used for clinical injection and can ensure that the medication safety cannot be lowered while the stability of products is improved.
Owner:SICHUAN CREDIT PHARMA +1
Sterility test method for gentamicin sulphate injection
ActiveCN110484596ASterility testSterility testing is accurate and reliableMicrobiological testing/measurementAgainst vector-borne diseasesEscherichia coliPositive control
The invention discloses a sterility test method for a gentamicin sulphate injection. A membrane filtration method is adopted, and the preparation step of a culture involves that a bigeminy closed membrane filter is used for membrane filtration, wherein the flushing amount of a buffer solution is 100-800 mL per membrane, and the addition amount of a culture medium is 100 mL per membrane; the preparation step of a test group involves that the gentamicin sulphate injection is added into a diluent to be diluted, wherein the volume of the diluent is 0-10 times of the volume of the injection; the filtration amount of the injection is 0.8 million of filtering titers per membrane; the preparation step of a positive control group involves that escherichia coli is adopted as positive contrast bacteria, and the positive contrast bacteria are added after the culture medium is added. According to the provided sterility test method for the gentamicin sulphate injection, according to the bacteriostatic characteristic of gentamicin sulphate, the sterility test process, the culture medium, the buffer solution and other elements are adjusted, and the sterility test can be conducted accurately and reliably on the gentamicin sulphate injection.
Owner:甘肃省药品检验研究院
Double-layer aseptic package, packaging method and sterility test method for large volume injections
InactiveCN104434517AGuaranteed sterilityExcellent Mechanical ShockMicrobiological testing/measurementPharmaceutical containersMoistureBiomedical engineering
The invention relates to the field of production of large volume injections, and provides a double-layer aseptic package, packaging method and sterility test method for large volume injections. The package comprises an inner packaging bag and an outer packaging bag wrapping the inner packaging bag. The space between the inner packaging bag and the outer packaging bag is vacuumized, so that the outer packaging bag is nearly attached to the inner packaging bag, and the space between the inner packaging bag and the outer packaging bag is aseptic. The mechanical performance and moisture blocking performance of the packaging bags are enhanced, and the problems of product pollution and medical potential safety hazard caused when packages leak or moisture evaporates in the transportation or storage process are solved. Besides, the vacuumized packaging bags are subjected to sterilization, liquid medicine in the inner packaging bag of the package is aseptic, and meanwhile the space between the inner packaging bag and the outer packaging bag is also aseptic. Thus, when the package is applied to the environment like clinical dispensing having high aseptic requirements, it is only required that the outer packaging bag is taken off, and medical staff do not need to disinfect packages one by one.
Owner:XIAN JINGXI SHUANGHE PHARMA
Disposable three-cavity gastroscope and enteroscope sleeve
InactiveCN103054541AImprove sterilityChange the disadvantagesSurgeryDiagnostic Endoscopic ProcedureControl system
The invention relates to the technical field of medical instruments, in particular to a disposable three-cavity gastroscope and enteroscope sleeve which can prevent cross infection during gastroscopy. The technical scheme includes that firstly, a negative pressure suction forceps channel tube and a water and air injecting pipe are fixed on the outer side of a flexible pipe, thereby an independent water and air pipeline system, an independent negative pressure suction and forceps channel system, a gastroscope air and water eduction control system and a negative pressure suction and forceps channel eduction control system are formed, the complete disposable three-cavity gastroscope and enteroscope sleeve is formed, and gastroscope or enteroscope can be thoroughly isolated from a gastral cavity or an intestinal tract during gastroscopy or enteroscopy. Accordingly, defects of prior built-in pipelines of the gastroscope and the enteroscope are overcome, sterility tests of the gastroscope and the enteroscope are achieved, and the cross infection is completely eradicated.
Owner:郑杰
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com