Method for storing endometrial stem cells
A technology of endometrial stem cells and cells, which is applied in the corresponding culture and storage technology field, and can solve problems such as ethical restrictions and difficulties in the source of stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
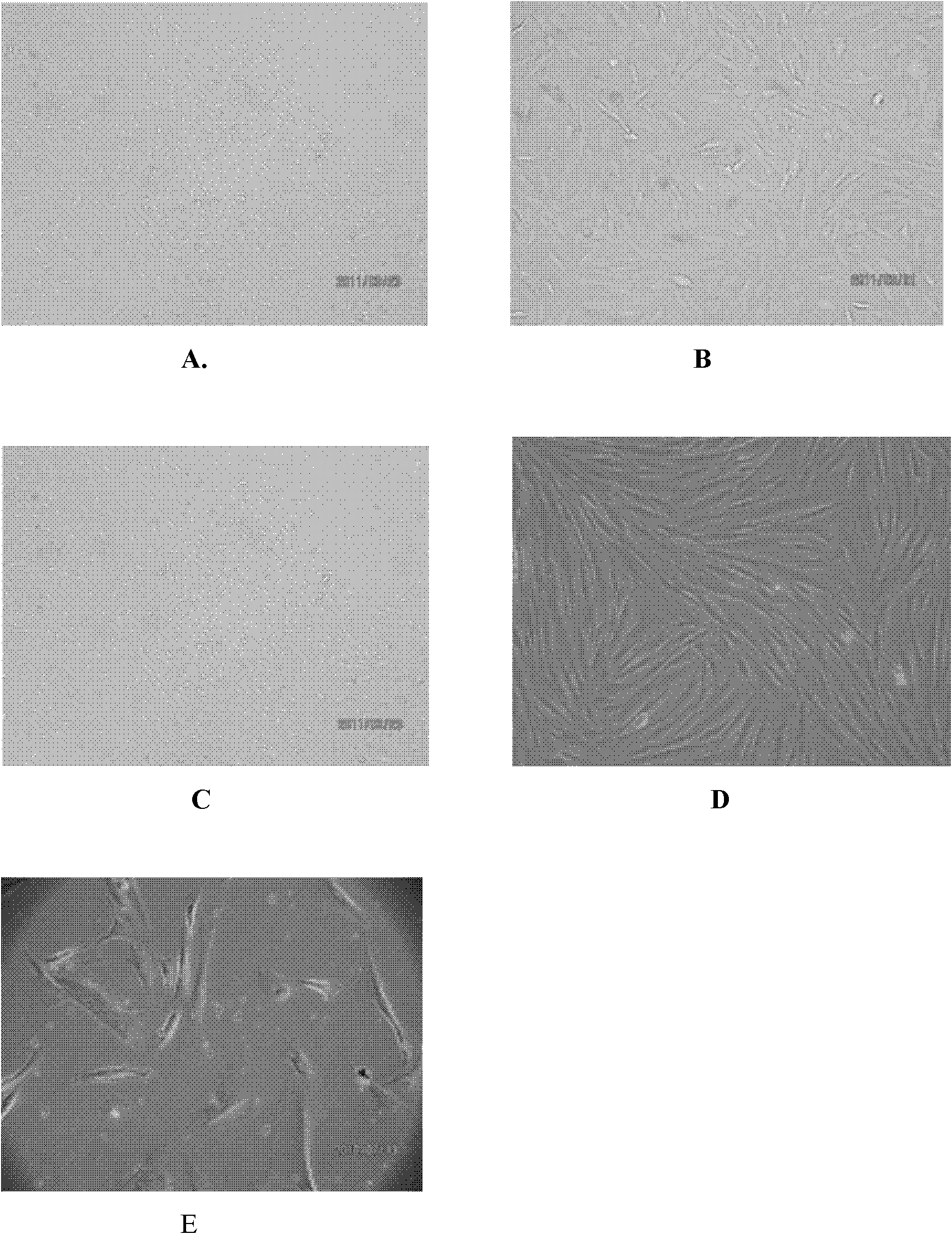
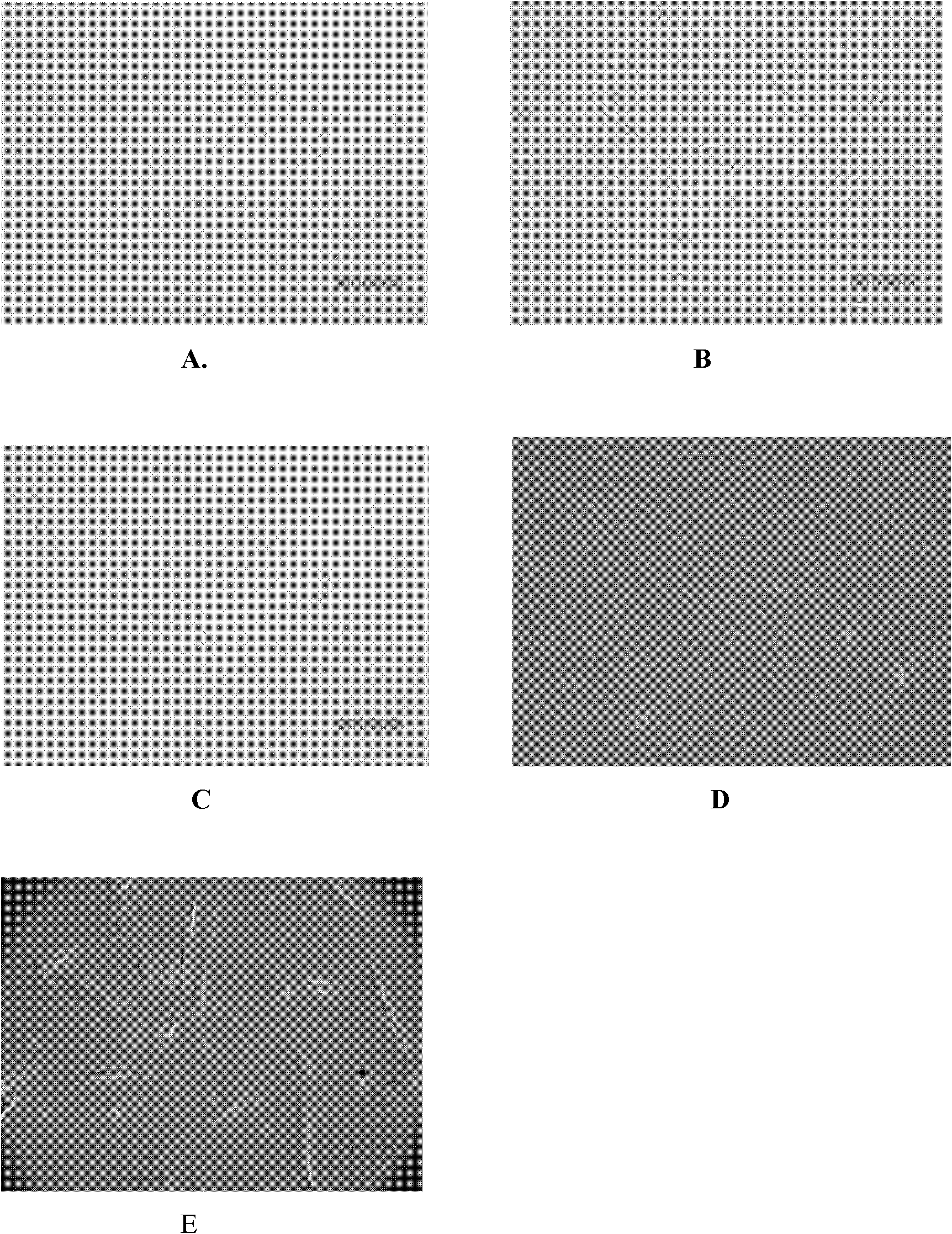
Embodiment 1
[0041] Example 1: A method for storing endometrial stem cells, that is, separation, culture, expansion, cryopreservation, and recovery of human endometrial stem cells; the following steps are performed in sequence:
[0042] 1). Prepare collection kit and preparation medium:
[0043] Collection tube 1: It contains 20ml Hank's Balanced Salt Solution (HBSS), and add the following ingredients to the following concentrations: Vancomycin (Vancomycin) 70μg / mL, Cephalexin (Claforan) 200μg / mL, Kanamycin (Amikacin) ) 100μg / mL, Gentamycin (Gentamycin) 140μg / mL, Amphotericin B (Amphotericin B) 2.5μg / mL and 400 units of heparin added.
[0044] Collection tube 2: filled with 10ml HBSS (Hank's balanced salt solution), and add 180 units of heparin.
[0045] Before starting the collection of menstrual blood in step 2), the collection tube 1 and collection tube 2 should be stored at 4°C.
[0046] Prepare Chang complete medium: under aseptic conditions, add 65mL MEM-alpha medium (MEM alpha, Invitrogen co...
Embodiment 2
[0082] Example 2. Cell resuscitation culture
[0083] The frozen sample obtained in Example 1 above was taken out of the liquid nitrogen storage tank and immediately put into a 37°C water bath to thaw. When it is observed that the ice core in the cryopreservation tube is only the size of a soybean, remove the cryopreservation tube and immediately put it into the ice-water mixture. Under aseptic conditions, gently aspirate the sample from the cryopreservation tube, add it to a 50mL sterile centrifuge tube, and immediately add 10mL of Chang complete medium, mix well, wash, and centrifuge at 4℃ and 1000rpm for 10 Minutes, remove the supernatant from the tube. Repeat the operation once. Use plate blue for cell counting and viability analysis. According to 10000 / cm 2 The density (using Chang complete medium) to inoculate the cells to 75 cm 2 In the culture flask, put CO at 37℃, saturated humidity, and 5% volume fraction 2 Culture in an incubator. According to the growth of the cel...
Embodiment 3
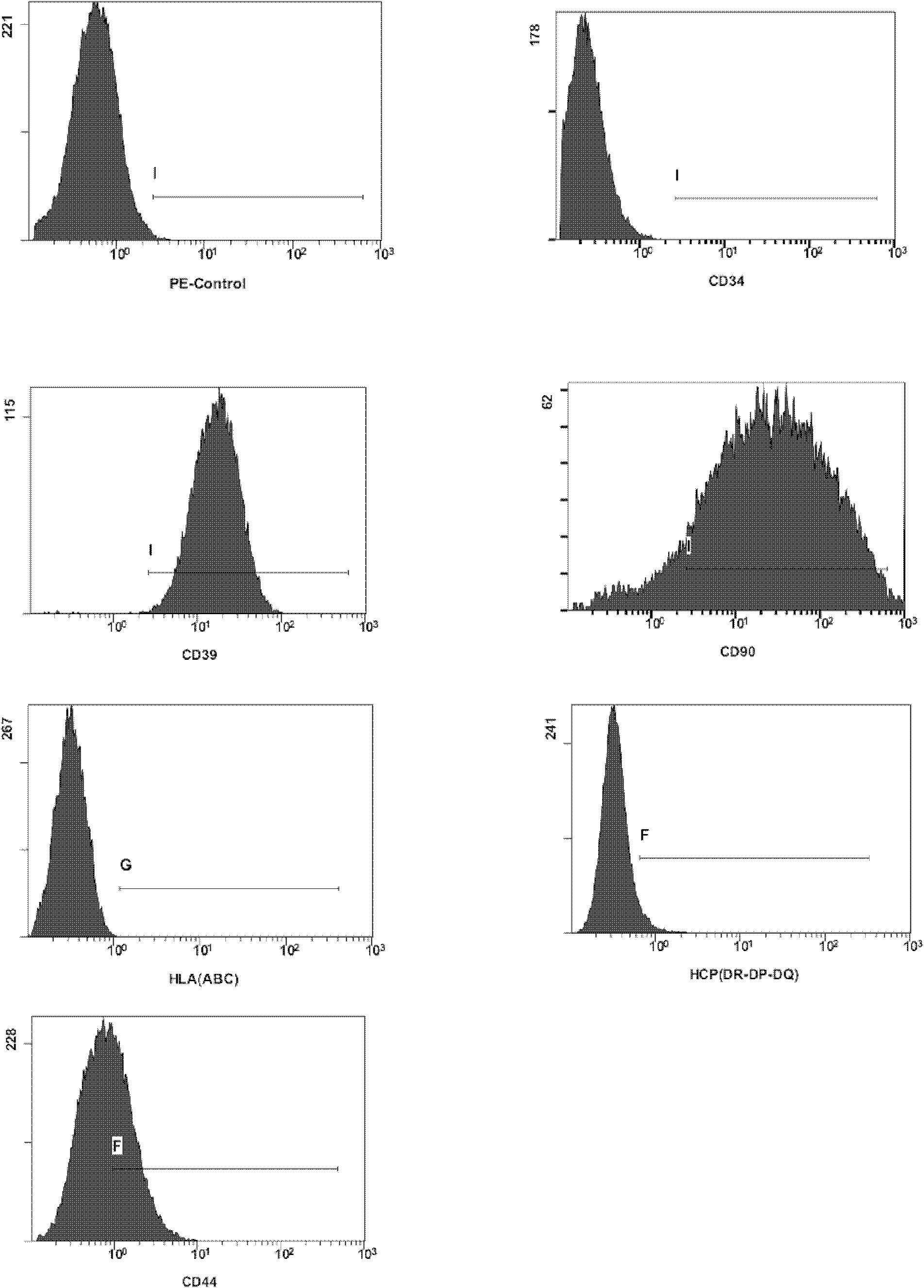
[0084] Example 3. Flow Cytometry
[0085] The endometrial mesenchymal stem cells cultured at generation P5 obtained in Example 1 were selected for flow cytometry as follows.
[0086] Each test tube contains a concentration of 5*10 6 / ml 2ml of P5 generation cells.
[0087] 1) Preparation of cell suspension:
[0088] Digest the cells with 1 ml of 0.25% (mass concentration) trypsin, centrifuge at 500 rpm for 5 min, and discard the supernatant. Wash twice with PBS, then resuspend the cells in 2ml PBS to make the cell concentration 5*10 6 / ml.
[0089] 2) Fixed with 4% paraformaldehyde (prepared in PBS) for 15 minutes, and then washed twice with PBS (1500 rpm, centrifugation for 5 minutes).
[0090] 3) 5% BSA is fixed for 40 minutes.
[0091] 4) According to the dilution requirement of flow cytometry antibody (direct standard) every 10 6 Add 20ul antibodies (CD39, CD44, CD90, CD34, HLA (ABC), HLA (DR-DP-DQ)) to each cell / 200ul, protect from light, and incubate at 37°C for 30 min.
[0092] 5) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com