Sterility test method for gentamicin sulphate injection
A gentamicin sulfate, sterility inspection technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve problems such as excessive flushing fluid volume, false negatives, inaccurate inspection, etc., to achieve accurate Reliable sterility check, reduced bacteriostatic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 1. A method for sterility inspection of gentamicin sulfate injection, the steps are as follows:
[0061] 1.1 Preparation of culture medium, bacterial liquid, diluent, and buffer: prepare thioglycolate fluid medium and tryptone soya peptone liquid medium; prepare Escherichia coli suspension with bacterial count less than 100 cfu / mL; prepare buffer Sodium chloride-peptone buffer at pH 7.0.
[0062] 1.2 Preparation of experimental group: After a group of double closed membrane filters were wetted with buffer, 10 bottles of gentamicin sulfate injection samples were filtered through, and each membrane was washed with 800 mL of buffer, 100 mL each time, to obtain 2 samples containing the sample. Filter cartridges; 100 mL of thioglycolate fluid medium and 100 mL of tryptone soy medium were added to the two filter cartridges, respectively, and cultured and observed daily.
[0063] 1.3 Preparation of positive control: After a set of double closed membrane filters were wetted wi...
Embodiment 2
[0078] The sterile inspection method and method applicability verification test of gentamicin sulfate injection are all referring to Example 1, the difference is: diluent 0.9% sterile sodium chloride solution is also prepared; gentamicin sulfate injection sample is filtered Add 100 mL of diluent to each 10 bottles before dilution; rinse each membrane with 500 mL of buffer, 100 mL each time.
[0079] The method applicability verification test results of the present embodiment are as follows:
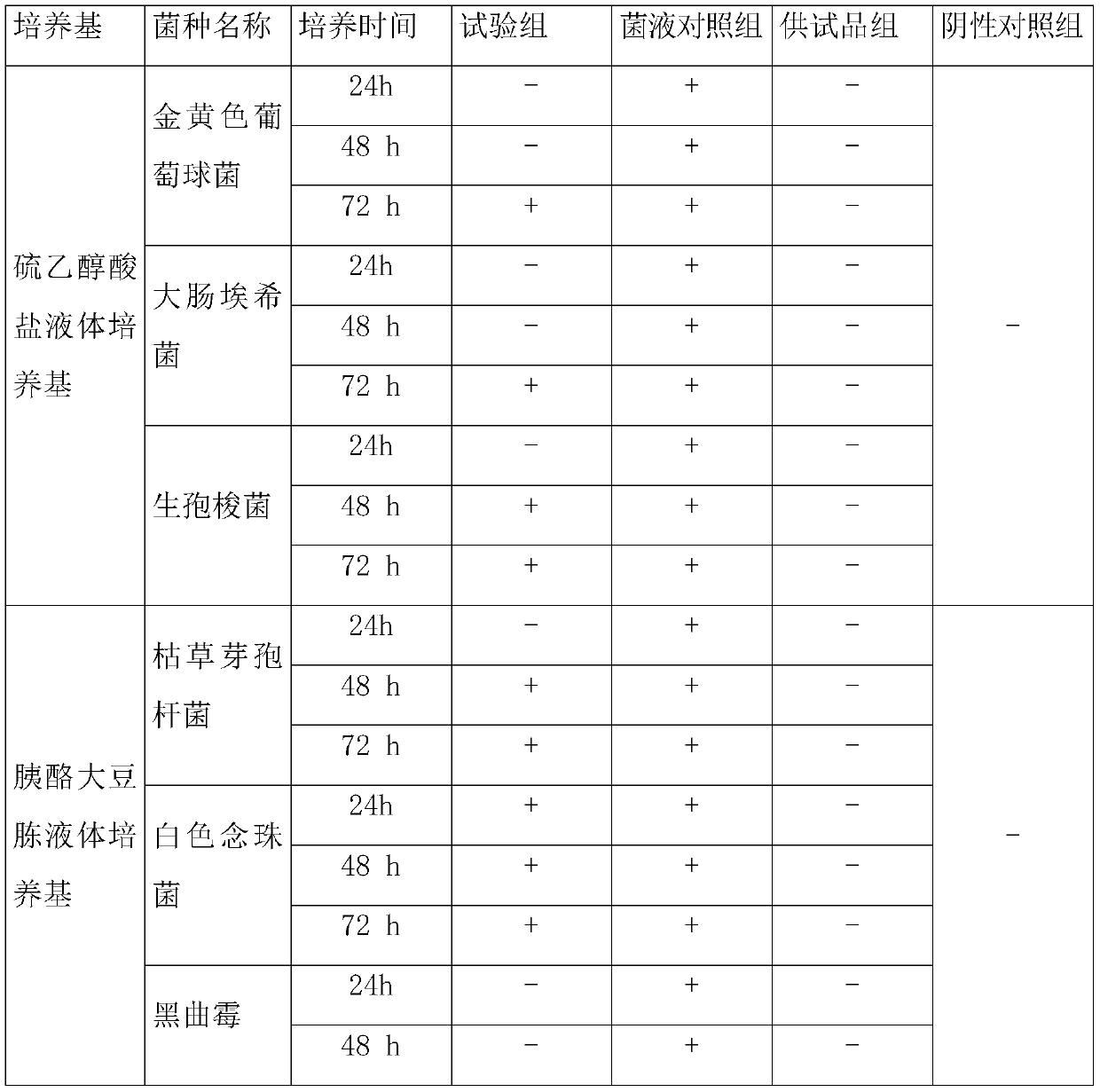
[0080] Table 2
[0081]
[0082] Note: + means the liquid is cloudy; - means the liquid is clear
[0083] The results in Table 2 show that 6 kinds of experimental bacteria all grow after culturing for 72 hours, indicating that the inspection method of Example 2 is suitable for sterility inspection of gentamicin sulfate injection.
Embodiment 3
[0085] The sterility inspection method of gentamicin sulfate injection and the verification test of method applicability are all referring to Example 1, the difference is: 200 mL of buffer solution is added to each 10 bottles of gentamicin sulfate injection as a diluent to dilute before filtration; Rinse each membrane with 300 mL of buffer, 100 mL each time.
[0086] The method applicability verification test results of the present embodiment are as follows:
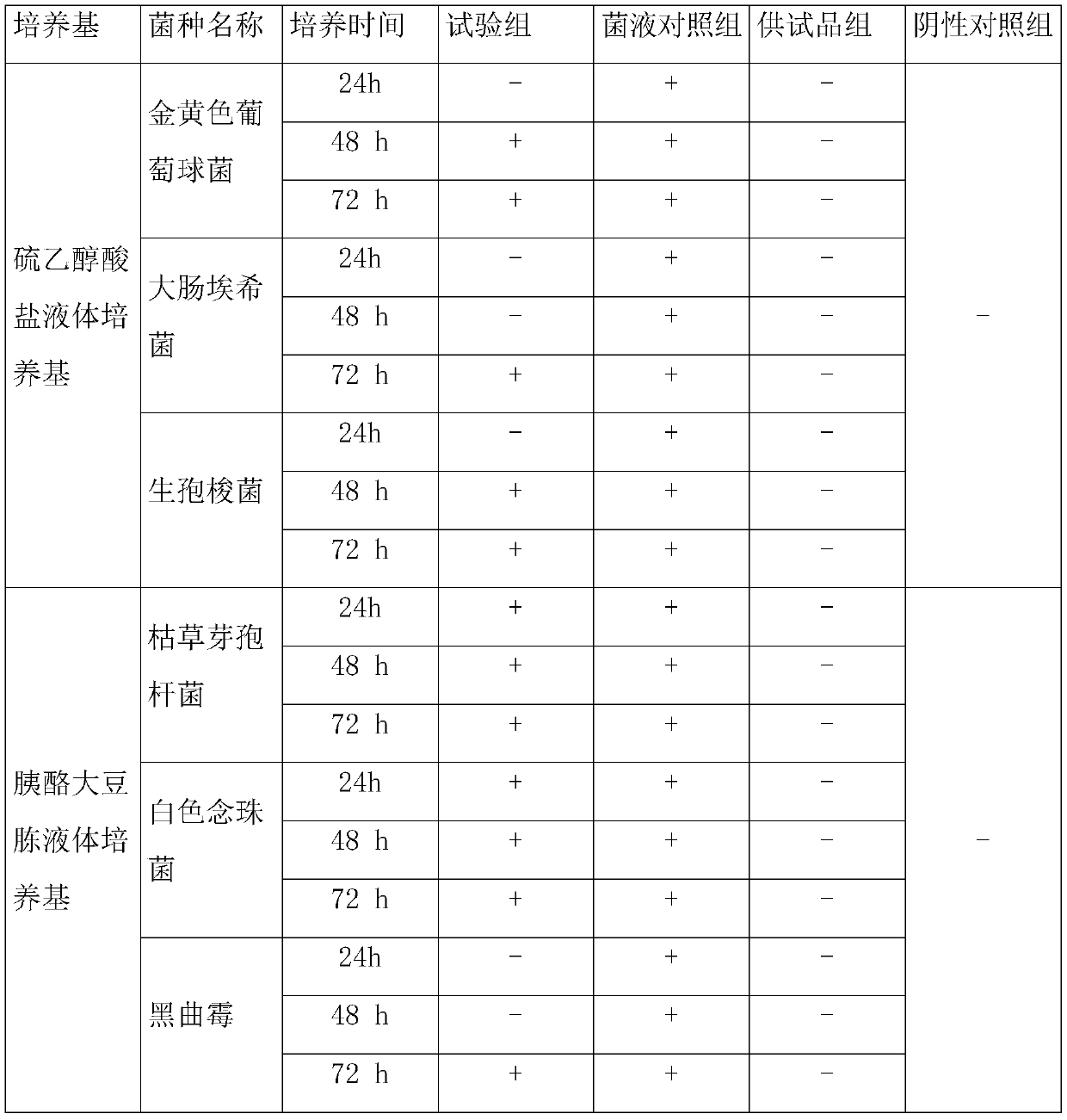
[0087] table 3
[0088]
[0089] Note: + means the liquid is cloudy; - means the liquid is clear
[0090] The results in Table 3 show that 6 kinds of experimental bacteria all grow after culturing for 48 hours, indicating that the inspection method of Example 3 is suitable for sterility inspection of gentamicin sulfate injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com