Sterility test method for antibiotic sustained-release pharmaceutical preparation
A slow-release drug, sterility inspection technology, applied in biochemical equipment and methods, microbial determination/inspection, etc. problems such as reports on the sterility inspection of drug-released pharmaceutical preparations, etc., to achieve the effect of reliable detection method and no effect on growth and survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
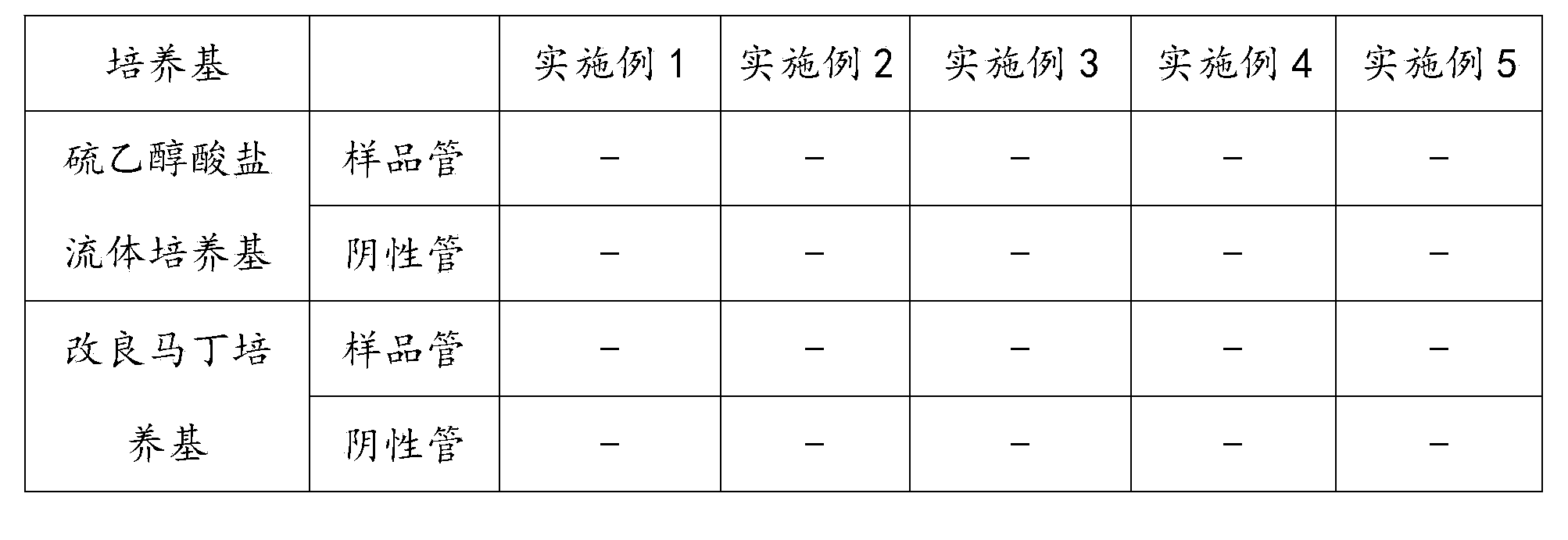
[0041] Take 10 samples of azithromycin sustained-release eye drops (2.5ml / bottle), draw 1.25ml for each, a total of 12.5ml into a sterile Erlenmeyer flask, add 80ml of 5% sterile calcium chloride (0.036mol) solution, cover plug. After shaking, let it stand for precipitation, and tilt the Erlenmeyer flask to make the precipitation form a gel mass. Pour the mixed solution together with the precipitate into a sterile filter, wash the mixed cellulose filter membrane (pore size 0.45 μm) with 100ml / time×10 times of 0.1% sterile peptone aqueous solution, and wash another mixed cellulose filter membrane in the same way ( Pore diameter 0.45μm). Put the filter membrane into 100ml of thioglycolate fluid medium and 100ml of modified Martin medium respectively, culture the thioglycolate fluid medium at 30-35°C, and cultivate the improved Martin medium at 23-28°C , cultivated for 14 days, and observed whether there was bacterial growth every day. Result: No apparent microbial growth. ...
Embodiment 2
[0043] Take 10 samples of azithromycin sustained-release eye drops (2.5ml / bottle) respectively, draw 1.25ml for each 12.5ml into a sterile conical flask, add 100ml of 5% sterile ferrous chloride (0.039mol) solution, cover plug. After shaking, let it stand for precipitation, and tilt the Erlenmeyer flask to make the precipitation form a gel mass. Pour the mixed solution together with the precipitate into a sterile filter, wash the mixed cellulose filter membrane (pore size 0.45 μm) with 100ml / time×10 times of 0.1% sterile peptone aqueous solution, and wash another mixed cellulose filter membrane in the same way ( Pore diameter 0.45μm). Put the filter membrane into 100ml of thioglycolate fluid medium and 100ml of modified Martin medium respectively, culture the thioglycolate fluid medium at 30-35°C, and cultivate the improved Martin medium at 23-28°C , cultivated for 14 days, and observed whether there was bacterial growth every day. Result: No apparent microbial growth. T...
Embodiment 3
[0045] Take 10 samples of levofloxacin sustained-release eye drops (2.5ml / bottle) respectively, draw 1.25ml for each 12.5ml into a sterile Erlenmeyer flask, add 200ml of 10% sterile zinc sulfate (0.125mol) solution, cap the . After shaking, let it stand for precipitation, and tilt the Erlenmeyer flask to make the precipitation form a gel mass. Pour the mixed solution together with the precipitate into a sterile filter, wash the mixed cellulose filter membrane (pore size 0.45 μm) with 100ml / time×10 times of 0.1% sterile peptone aqueous solution, and wash another mixed cellulose filter membrane in the same way ( Pore diameter 0.45μm). Put the filter membrane into 100ml of thioglycolate fluid medium and 100ml of modified Martin medium respectively, culture the thioglycolate fluid medium at 30-35°C, and cultivate the improved Martin medium at 23-28°C , cultivated for 14 days, and observed whether there was bacterial growth every day. Result: No apparent microbial growth. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com