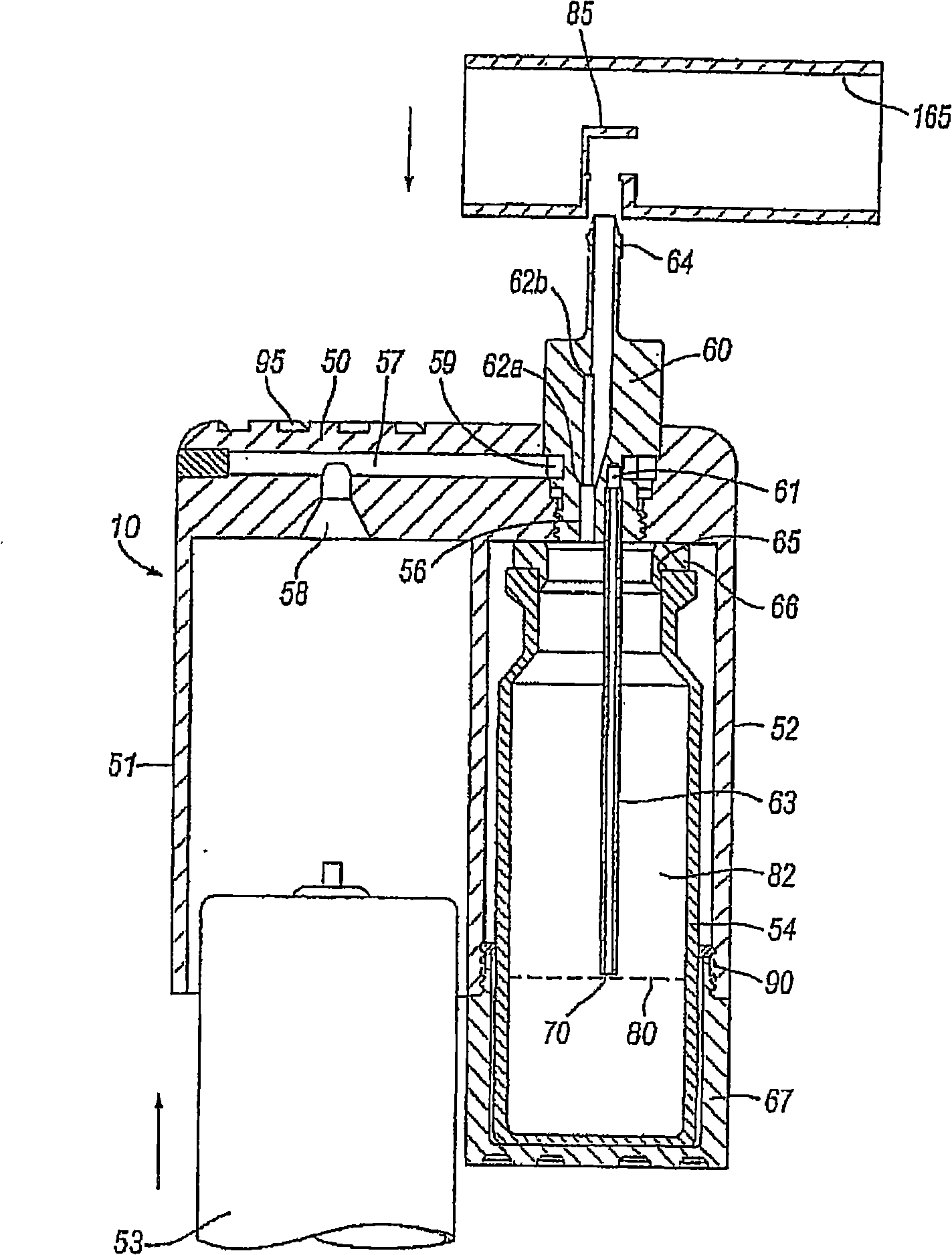
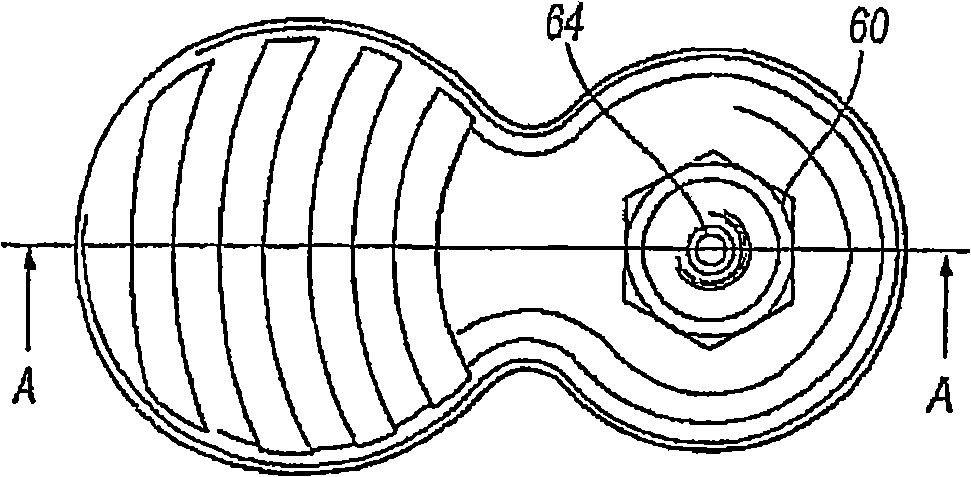
Delivery device for a powder aerosol
A delivery device, aerosol technology, applied in the direction of nebulizer for treatment, medical device, inhaler, etc., can solve the problems of low dose and inapplicability, and achieve the effect of improving the problem of patient coordination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] The device according to the invention has been successfully used in the experimental veterinary treatment of respiratory disturbances in horses using Pumatane, as detailed below.
[0091] Horses are prone to a plethora of respiratory distress. Asthma is comparable to equine asthma, and both diseases share similar etiology and pathology. The disease has been shown to occur via a Th2 cytokine-driven mechanism in equines (Lavoie, J-P., Maghni, K., Desnoyers, M., Taha, R., Martin, J.G., and Hamid Q.A. (2001)) . Neutrophilic airway inflammation in horses with asthma is characterized by a Th2 cytokine profile (Am. J. Respir. Crit. Care. Med 164 1410-1413). Like their human counterparts, they have poor ease of use and have an estimated 2 range of enormous lung surface area.
[0092] The goal of this study was to investigate the use and delivery of thermally labile, hygroscopic anhydrous surfactants while ensuring acceptable physicochemical properties. The surfactant used ...
Embodiment 2
[0115] Study using Pumatan as a model drug figure 1 Performance of the inhaler shown in . Specifically, the effect of loading dose on dry powder delivery and the effect of tank pressure on atomization efficiency were investigated.
[0116] Reported clinical studies required a 4H 100 mg, 8 h 30 min dosing regimen prior to allergen challenge [Babu, KS. et al, ibid]. Such high doses were well tolerated, and in all cases early asthmatic responses were resolved. However, due to the proximity of pumatant to endogenous (endogenous) surfactants (eg, low transition temperature and high wet affinity), the energy required to atomize the powder cannot be achieved using conventional means.
[0117] Physical properties of Pumatan
[0118] Prior to in vitro testing, micronized pumatant was first characterized in terms of particle morphology, size distribution, hygroscopicity, and crystal structure.
[0119] The particle morphology of micronized pumatant (Jeol 6310: Jeol, Japan) was studi...
Embodiment 3
[0175] using a method called Zofac TM A device 300 of dry powder medicament was studied. Notably, a single actuation from a loading dose of approximately 100 mg of Zofac TM Deliver up to approximately 40 mg of Zofac in aerosol form in TM . Zofac TM It is composed of two synthetic phospholipids, dipalmitoylphosphatidylcholine (DPPC) and unsaturated phosphatidylglycerol (PG), at a ratio of 7:3. Zofac TM Has a mass median aerodynamic diameter of less than 5 microns. Zofac TM Contains not more than 4% moisture by weight. Of course, other dry powder medicaments can also be used with the device 300 .
[0176] The properties of the aerosol delivered via the device 300 were evaluated by Malvern laser diffraction and Andersen cascade impactors. The power source 305 is a nitrogen tank at 10 bar or 14 bar. Zofac delivered from a 100 mg loading dose at 10 bar and 14 bar is shown in Table 4 TM amount. It was observed that higher doses were delivered with the nitrogen tank at 14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com