Transglutaminase mediated conjugation of growth hormone
A technology of glutamine and growth hormone, applied in the field of post-translational binding of growth hormone, can solve problems such as limiting connection points and restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
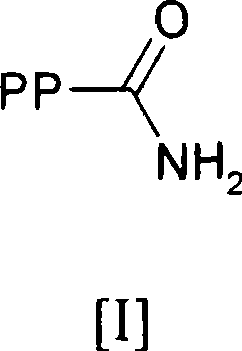
[0517] Embodiment 1. A method of covalently linking PEG to a polypeptide comprising at least one glutamine residue, said method comprising in one or more steps making said glutamine residue comprising a polypeptide represented by formula [I] Aminoamide residues
[0518]
[0519] where PP means that by removing -C(=O)-NH from the side chain of a glutamine residue present in the polypeptide 2 The obtained polypeptide group, with the nitrogen-containing nucleophile of formula [II]
[0520] h 2 N-D-R-X
[0521] [II]
[0522] Where D represents -O- or a single bond;
[0523] R for C 1-6 Alkylene, -(CH 2 ) 4 -CH(NH 2 )-CO-NH-CH 2 -, -(CH 2 ) 4 -CH(NHCOCH 3 )-CO-NH-CH 2 - or C 5-15 Heteroalkylene;
[0524] X stands for -O-NH 2 , aldehydes, ketones, or can be converted to -O-NH by further reactions 2 , potential groups of aldehydes or ketones;
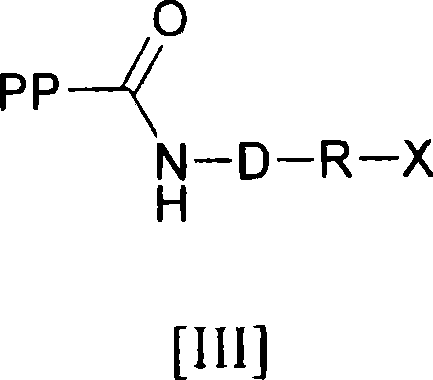
[0525] Reaction in the presence of transglutaminase, thereby forming the transaminated polypeptide of formula [III]
[...
Embodiment approach 2
[0545] Embodiment 2. The method according to embodiment 1, wherein D represents -O-.
Embodiment approach 3
[0546] Embodiment 3. The method according to embodiment 1, wherein D represents a single bond.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com