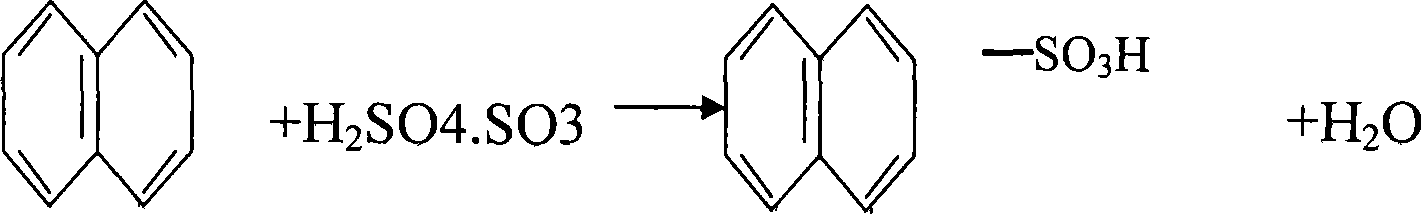
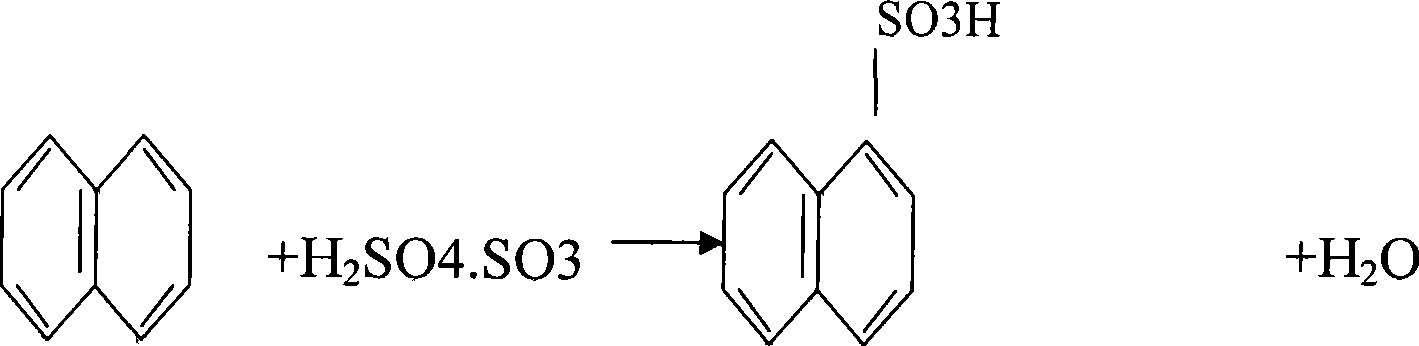
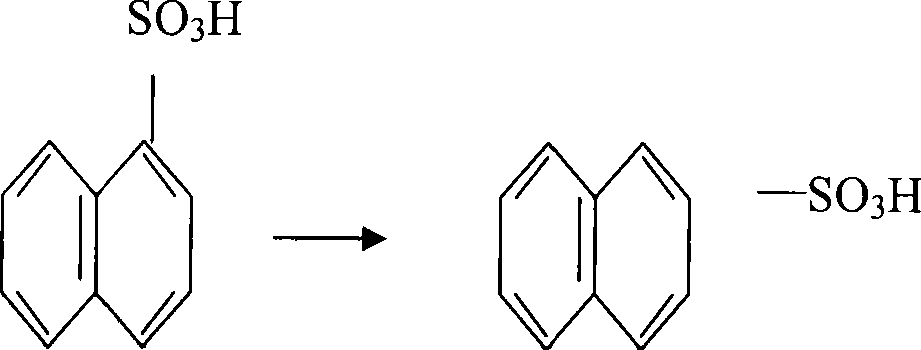
105% acid sulfonation manufacturing technique of 2-naphthalenol
A production process and acid sulfonic acid technology, applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems of low total yield of sulfonation reaction, hinder substitution reaction, etc., reduce the production of other by-products of naphthalene series, and improve sulfonation The effect of conversion and product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The 2-naphthol 105% acid sulfonation production process is to put the molten refined naphthalene into the reactor at one time, and after the temperature in the reactor is raised to 135-140 ° C, the mixture of naphthalene and 105% oleum is 1. Add 105% oleum at a mass feeding ratio of 1.28:1, and the feeding time is 30 minutes; after the 105% oleum is added, the temperature in the reactor rises to 160°C. After the reaction is completed, take a sample to analyze the acidity. 24-27% is qualified. If the sampling is unqualified and the acidity is high, add naphthalene. Naphthalene is added dropwise at a temperature of 160°C. Sampling is performed while adding. If the acidity is low, the acid is supplemented, and the acid supplement is added dropwise at a temperature of 160°C. Sampling and analysis is performed while the acid is added, and the material is pressed after passing the test. Transfer to the next process until the sampling is qualified.
[0020] Give a concrete e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com