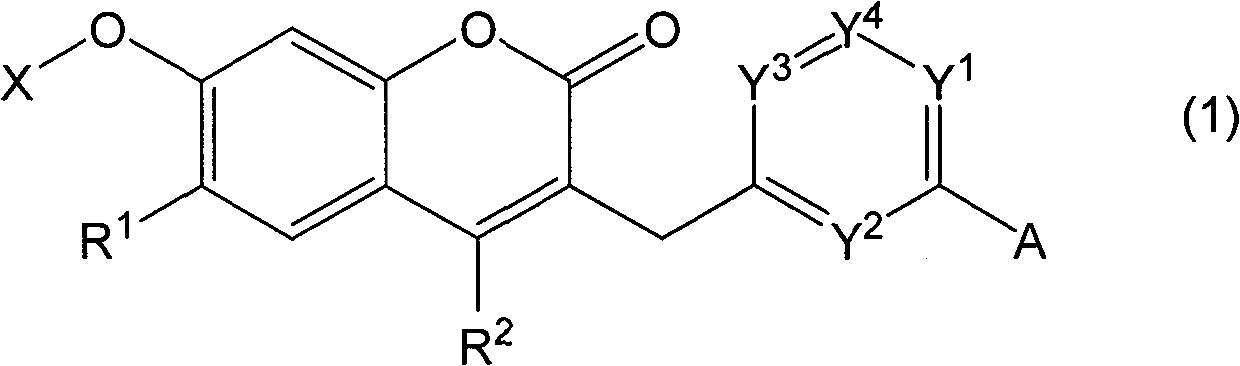
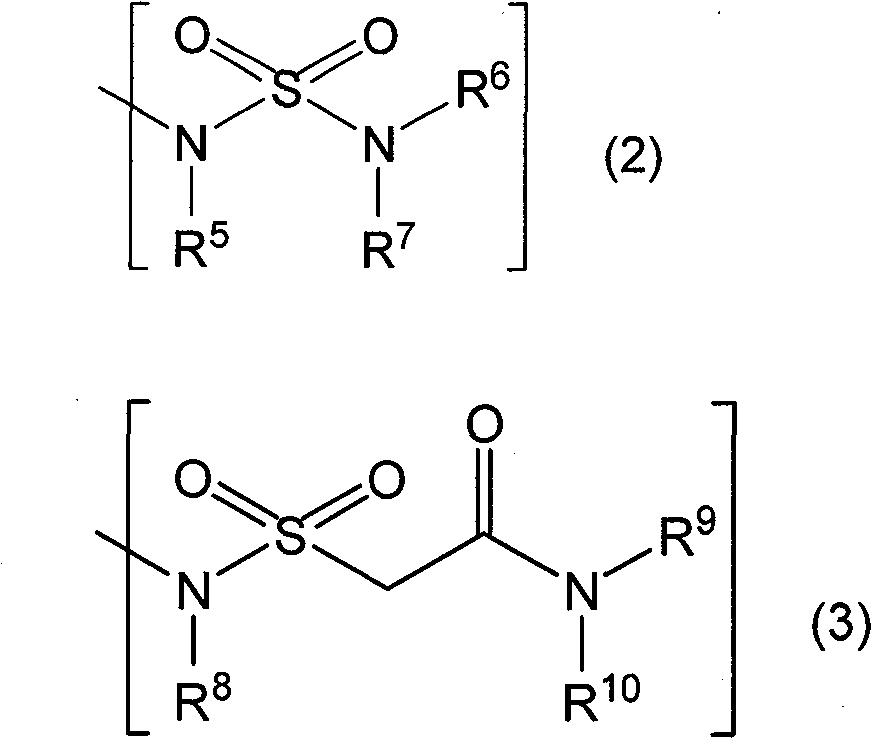
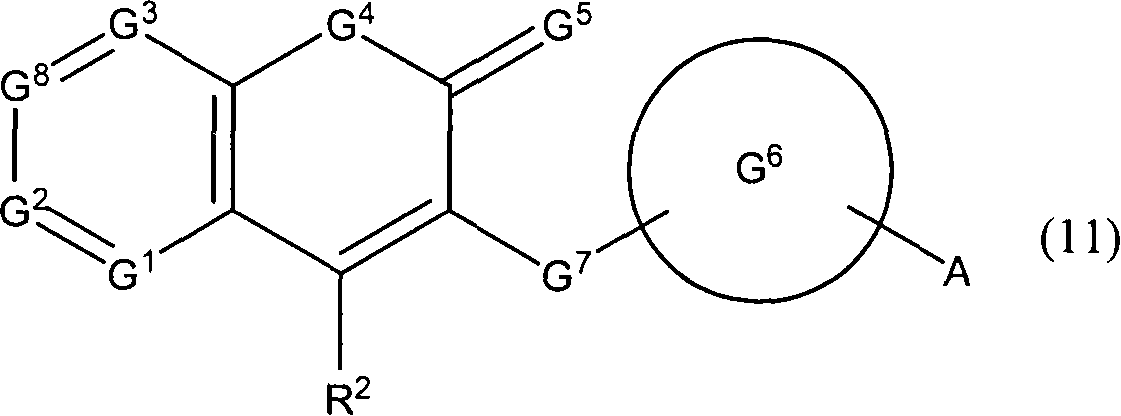
Novel coumarin derivative having antitumor activity
A compound, methyl technology, applied in the field of novel coumarin derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example
[0659] In the following production examples (synthesis examples), NMR analysis was performed using JNM-EX270 (270 MHz) manufactured by JEOL Corporation, JNM-GSX400 (400 MHz) manufactured by JEOL Corporation, or ARX-300 (300 MHz) manufactured by Bruka Corporation, and the NMR data were expressed in ppm (parts permillion, δ) are shown, referenced to the deuterium lock signal from the sample solvent.
[0660] In addition, mass spectrum data were obtained using JMS-DX303 manufactured by JEOL, JMS-SX / SX102A manufactured by JEOL, or a micro mass spectrometer (Navigator manufactured by Finningan) equipped with Agilent 1100 gradient high performance liquid chromatography manufactured by Agilent Technologies.
[0661] In the case of using a commercially available reagent, it was directly used for the reaction without performing pretreatments such as distillation and recrystallization. As a reaction solvent, when a commercially available solvent is used, an anhydrous solvent is used.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com